Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Low-Cost Protection For Biomass-Processing Catalysts
Renewables: Carbon film protects, stabilizes petrochemical catalysts
by Mitch Jacoby
November 30, 2012
| A version of this story appeared in
Volume 90, Issue 49
\
\
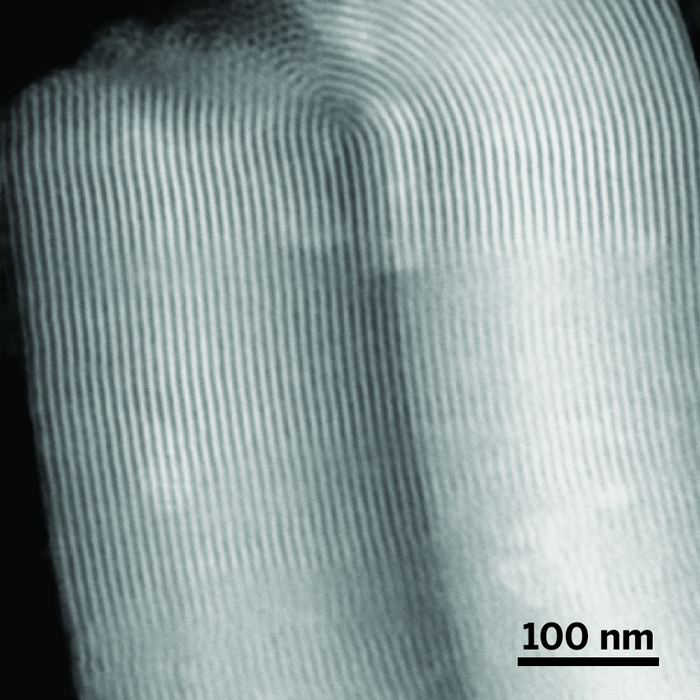
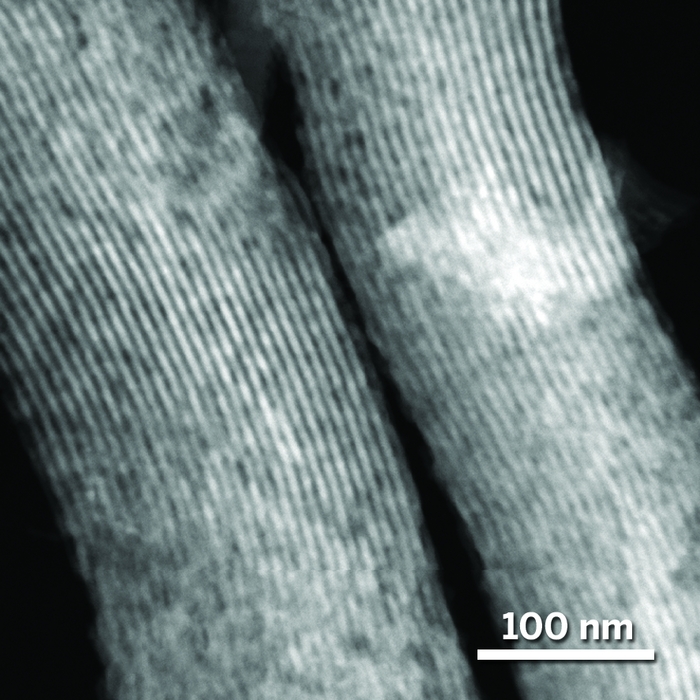
The well-ordered internal structure of SBA-15 collapses upon prolonged exposure to water at high temperature. But samples coated with a thin carbon film (top) remain unchanged even after hours of exposure (bottom).
Suitable catalysts for converting biomass to chemicals and fuels at low cost are not generally available today. A lot of petrochemical refining catalysts could do the job—but biomass refining conditions destroy them.
Researchers have now found a way around this problem, by enhancing the stability and durability of the supports on which catalytic metals are dispersed (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201206675).
Alumina and silica—and other oxide materials widely used to support metal catalysts for petroleum refining—work well under gas-phase reaction conditions. But transforming biomass-derived compounds, such as carbohydrates and other highly oxygenated compounds, generally calls for high-temperature and high-pressure liquid-phase aqueous conditions.
Those conditions often ruin porous oxide supports, which make petroleum refining catalysts unsuitable for biomass refining. For example, γ-alumina, a high-surface-area material, undergoes a phase change near 200 °C to the low-surface-area boehmite phase. And the well-ordered internal pore structure of SBA-15, a common form of silica, collapses when exposed to liquid water at that temperature.
A research team, including Hien N. Pham, Amanda E. Anderson, and Abhaya K. Datye of the University of New Mexico, has found that the supports can be protected by coating them with a thin carbon layer, which the team forms by decomposing simple sugars such as sucrose. The group, which also includes coworkers at Iowa State University, showed that a carbon film wards off most or all of the structural damage caused by exposing uncoated samples to high-temperature water.
They also conducted preliminary catalysis tests based on selective hydrogenation of acetylene to ethylene, a key industrial reaction, to determine whether the coated oxides are not just hydrothermally durable but also retain the same abilities as uncoated oxides to serve as catalyst supports. Indeed, they found that Pd-based catalysts on coated supports outperformed those on uncoated supports. Now the team is preparing to use the coated materials to study biomass conversion.
Describing the work as “an elegant and relevant study,” catalysis specialist Bert M. Weckhuysen of Utrecht University, in the Netherlands, says the carbon-coating approach is a clever way to make common oxides compatible with the demanding aqueous-phase conditions needed for processing biomass. “The study provides clear leads for producing a new generation of more stable catalytic solids,” he adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter