Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
X-raying Catalysts In Action
Tomography method monitors changes in chemically distinct nanoscale regions of catalysts under reaction conditions
by Mitch Jacoby
December 10, 2012
| A version of this story appeared in
Volume 90, Issue 50
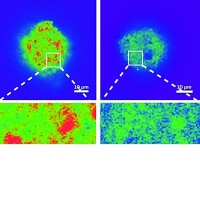
Thanks to a specially designed reactor cell, researchers can now probe changes in the chemical composition, morphology, porosity, and other properties of individual 20-μm-sized catalyst particles as the particles mediate chemical reactions (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201204930). The study conducted by Joy C. Andrews of SLAC National Accelerator Laboratory; Bert M. Weckhuysen of Utrecht University, in the Netherlands; and coworkers demonstrates a procedure for exploiting high-energy X-ray microscopy to penetrate deeply into complex materials and resolve chemically distinct nanoscale regions within their bulk. Solid catalysts often undergo substantial changes—sometimes beneficial, sometimes detrimental—upon exposure to reactive chemicals at high temperature and pressure. Mapping those changes in three dimensions as they occur could lead to improved catalysts, but capturing the information remains challenging. To demonstrate the new method’s capabilities, the team probed a model Fischer-Tropsch C–C coupling catalyst as it was exposed to 10 atm of a mixture of H2 and CO at 350 °C. The method pinpointed regions rich in Fe2O3, Fe2TiO5, Fe3O4, ZnO, and K2O and monitored the evolution of those regions over the course of several hours.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter