Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Edges Ease Surface Oxidation Of Metals
Metal atoms detach from defects to react with adsorbed oxygen
by Jyllian Kemsley
December 17, 2012
| A version of this story appeared in
Volume 90, Issue 51
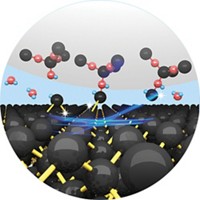
Oxidation of metal surfaces preferentially occurs when metal atoms detach from defect edges and react with adsorbed oxygen atoms, according to a report in Physical Review Letters (DOI: 10.1103/physrevlett.109.235502). The formation of oxide layers on metal surfaces can be corrosive, protective, or catalytically necessary, depending on the application. By using transmission electron microscopy to study oxidation of copper surfaces under low-pressure oxygen, a research group led by Guangwen Zhou of the State University of New York, Binghamton, determined that oxidation occurs as oxygen adsorbs onto the surface and reacts with copper atoms that detach from step edges and diffuse across the surface. Previous studies of surfaces with fewer defects—conducted under ultrahigh vacuum—had indicated instead that oxide layer growth requires significant bulk diffusion of oxygen atoms down into the metal lattice and metal atoms up to the surface. Zhou and colleagues found that oxidizing a flat surface leads to more stress and mechanical breakdown between the metal and oxide layers, while step-facilitated oxidation reduces stress and smooths the surface as metal atoms are drawn from the edges.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter