Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Gold Catalyst Approaches Performance Of Conventional Precious-Metal Catalysts
Small clusters of three to ten gold atoms catalyze alkyne hydration with industrial-scale turnover numbers and frequencies
by Sarah Everts
December 17, 2012
| A version of this story appeared in
Volume 90, Issue 51

Gold may be highly desired by commodity traders and jewelry buyers, but the metal’s popularity in the chemical industry has been limited by its modest abilities as a catalyst. Now, researchers in Spain report a gold catalyst that performs alkyne hydration with turnover or substrate conversion numbers on the order of about 10 million and turnover frequencies of some 100,000 per hour. The values approach those for catalytic metals used more commonly in industry, such as platinum and palladium (Science, DOI: 10.1126/science.1227813).
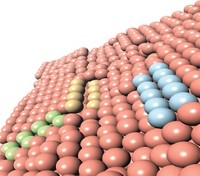
Curiously, the catalysis is performed by clusters of three to 10 gold atoms. The assemblies are larger than the catalytic species involved in homogeneous or single-phase catalysis and smaller than the nanosized clusters of dozens of atoms involved in heterogeneous or mixed-phase catalysis.
The results suggest that the catalysis community will “need to rethink the classical division between homogeneous and heterogeneous catalysis,” says chemist Avelino Corma, who carried out the study with colleagues at the Institute of Chemical Technology, in Valencia.
For true industrial uses, however, the catalytic gold clusters will first need to be stabilized because they are currently short-lived, Corma says. The catalyst will also have to show good performance in industrial-scale reactions. His team is working on this now, he adds.
“It’s very thought-provoking work,” says Steven P. Nolan, an expert in homogeneous catalysis at St. Andrews University, in Scotland. Because the reported catalyst is in a previously unknown sweet spot between the scale of heterogeneous and homogeneous catalysts, researchers may have to reconsider the nature of gold species currently believed to be involved in other gold-catalyzed reactions, Nolan says. For example, degradation or rearrangement products of putative gold catalyst species may be the catalytically active agents in those reactions, he says.
In addition to sparking efforts to understand the mechanism of such reactions, the new work might eventually push gold catalysis into the industrial big leagues. “The results will open the door for future industrial applications beyond fine chemicals, which are typically produced in smaller scale,” notes a commentary by Heidelberg University chemist A. Stephen K. Hashmi (Science, DOI: 10.1126/science.1231901).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter