Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
The Cholesterol Bet
Taking lessons from a failed drug, firms are ready to test a heart health hypothesis
by Carmen Drahl
February 20, 2012
| A version of this story appeared in
Volume 90, Issue 8

Dec. 2, 2006, was a day drugmakers won’t soon forget. On that day, Pfizer, the world’s biggest drug company, faced devastating news: Its highest profile drug candidate, the cholesterol-targeted molecule torcetrapib, had increased the risk of death in a 15,000-patient clinical trial. In light of the data, Pfizer promptly pulled the plug on the cholesteryl ester transfer protein (CETP) inhibitor that already had cost more than $800 million to develop. The torcetrapib news rocked the cardiovascular research field and left Pfizer without a potential new medication to supplement the blockbuster cholesterol drug Lipitor (atorvastatin), which was careening toward the patent cliff.
“I’ve never seen a set of data that had such a huge impact,” says James A. Sikorski, an independent medicinal chemistry consultant who previously worked on cardiovascular-disease-fighting agents at Searle, Pharmacia, and Georgia start-up AtheroGenics. “The whole field just came away wondering what the future of developing drugs for cardiovascular disease would be,” he says. “People were wondering how companies would respond.”
Some companies stopped research on CETP inhibitors. But not all of them did. Today, three CETP inhibitors are in late-stage clinical trials: Merck & Co.’s anacetrapib, Roche’s dalcetrapib, and Eli Lilly & Co.’s evacetrapib. They remain candidates because in the years since torcetrapib cast a shadow on cholesterol drug discovery, researchers have looked into what happened and found biochemical warning signs to watch. None of those warning signs have been reported for any of the new CETP blockers. And experts are awaiting data from clinical trials of CETP inhibitors on tens of thousands of people, which will give them an indication of whether blocking CETP will translate into a lower risk of heart attacks or strokes.
Researchers think blocking CETP is beneficial because it raises levels of high-density lipoproteins (HDLs), the so-called good cholesterol that doctors monitor at every checkup. The clinical trials could reveal just how good an idea it is to raise patients’ good cholesterol levels.
HDL has a good reputation because it transports cholesterol away from the arteries and to the liver, which experts think prevents cholesterol clogs on artery walls. But HDL is just one player in a complex lipoprotein system. Another player is the lipid transfer protein CETP. The liver secretes CETP into the bloodstream, where it brokers cargo trades among the various lipoproteins, including low-density lipoprotein (LDL), the so-called bad cholesterol. For example, CETP helps exchange the cholesteryl esters in HDL for the triglycerides in LDL.
Studies of animals, as well as people lacking CETP, formed much of the early basis for pursuing CETP inhibitors as drugs (Arterioscler. Thromb. Vasc. Biol., DOI: 10.1161/01.ATV.0000054658.91146.64). However, “different studies have concluded different things,” says Columbia University’s Alan R. Tall, a pioneer of HDL and CETP research.
For example, epidemiological studies in Japan during the 1980s and 1990s turned up pockets of people with a mutation that led to CETP deficiency. Those people all wound up with elevated levels of HDL, which might seem like a good thing automatically, and for the most part, it was. But some people without CETP would still develop cardiovascular diseases, Tall explains.
Researchers have studied CETP activity in rabbits as well as mice. Though results from mice were inconsistent, studies of rabbits consistently showed benefits from blocking CETP. It correlated with high HDL levels and reduced atherosclerosis, the thickened artery walls that result from cholesterol buildup and are a warning sign of cardiovascular trouble.

The appeal of CETP as a drug target goes beyond epidemiology and animal studies. Despite the success of statin drugs, which lower levels of LDL, cardiovascular disease is still the leading cause of death worldwide, according to the World Health Organization. Lowering the residual risk of heart disease is what drives researchers to examine other aspects of the cholesterol picture, including HDL and CETP, says Sanjay Kaul, a cardiologist at Cedars-Sinai Medical Center in Los Angeles. “You can only reduce the risk of major vascular events with statin therapy by about 25%,” Kaul says. “That means the glass is three-fourths empty, so to speak.”
What’s more, statins aren’t totally benign, says Rockefeller University physician-scientist Jan L. Breslow. As many as one in five people experience side effects from statin therapy, such as muscle fatigue, he says. People likely to have statin side effects in the real world—including older people, women, and patients with complex medical conditions—have been underrepresented in statin clinical trials, adds Breslow, who has no financial or consulting ties to drugmakers. For those patients, another cholesterol management strategy would be welcome, he says.
Excessive LDL isn’t always a patient’s only cholesterol management problem, adds Dara K. Lee, a cardiologist at New Mexico’s Presbyterian Heart Group. In New Mexico and elsewhere, she says, patients tend to have low levels of HDL and high levels of triglycerides, another set of hydrophobic molecules that at high levels have been linked to atherosclerosis but that aren’t easily controllable with statins. “We need another agent that will work safely with statins to raise HDL and lower triglycerides,” she says.
In the early- and mid-2000s, many hoped that torcetrapib would be that agent. Those hopes quickly unraveled once the news broke from Pfizer’s ill-fated 15,000-patient trial, called ILLUMINATE. Instead, researchers were left scrambling to figure out what happened with torcetrapib and what might prevent another CETP catastrophe.
During early-stage clinical trials of torcetrapib, Pfizer researchers learned that the drug led to a slight increase in blood pressure. The consensus among experts inside and outside the company at the time was that the health consequences of a small increase in blood pressure would be balanced by torcetrapib’s good-cholesterol-raising benefits, says Bruce H. Littman, president of Connecticut-based consulting firm Translational Medicine Associates and former vice president of global translational medicine at Pfizer. So Pfizer decided to advance torcetrapib to the Phase III clinical trial that would prove its undoing.
The small side effect turned out to be the tip of an iceberg, Littman says. He thinks Pfizer should have given greater consideration to backup CETP inhibitor candidates that didn’t increase blood pressure.
Commercial reasons and a legitimate medical need for more cholesterol-regulating options also factored into Pfizer’s decision, Littman adds. Because of the looming Lipitor patent expiry, “there was a lot of pressure to move torcetrapib forward,” Littman says. Changing to a backup candidate “made sense from a scientific point of view, but got trumped by commercial and human health points of view,” Littman explains. But it “would’ve cost maybe a one-year hit on the timeline,” too long, he adds, given the situation.
Pfizer “was under pressure, and they took their best shot at the problem,” agrees Tall, who consults for Pfizer, Merck, and Roche. Still, Pfizer “could have been more diligent in seeking to understand the mechanism of the blood pressure increase” during early-stage clinical trials, he says.
In the wake of their failed torcetrapib trial, the ILLUMINATE investigators began to look for answers. With Tall and others they crunched the numbers from patients’ blood work. They found several factors linked to an increased risk of death in the study: reduction in potassium levels; increases in sodium and bicarbonate levels; and an increase in aldosterone, a hormone made by the adrenal glands that raises blood pressure. Taken together, the findings meant that torcetrapib’s problems were due to off-target effects, the investigators suggested (N. Engl. J. Med., DOI: 10.1056/NEJMoa0706628).
Drug companies still developing CETP inhibitors also sought answers, as well as safety reassurance for their own products. When torcetrapib tanked “it was unclear whether the toxicity observed was related to the mechanism or the molecule,” says Yale Mitchel, vice president in the cardiovascular disease department at Merck Research Laboratories.
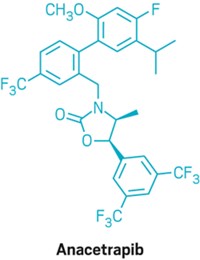
Merck slowed down development of its CETP inhibitor, anacetrapib, to clarify the cause of toxicity. With preclinical models, Mitchel says, Merck demonstrated that the blood pressure and aldosterone increases seen with torcetrapib were off-target effects and not seen with anacetrapib (Br. J. Pharmacol., DOI: 10.1038/bjp.2008.229). Anacetrapib also had no effect on aldosterone or blood pressure in humans, Mitchel says.
Merck researchers also delved into the basic biology of CETP and HDL. They determined how anacetrapib, torcetrapib, and Roche’s dalcetrapib inhibit CETP—by promoting formation of a complex between CETP and HDL (J. Lipid Res., DOI: 10.1194/jlr.M007468). And with Tall, Merck determined that the HDL from patients taking anacetrapib had similar or improved function compared with their HDL before receiving the drug (Arterioscler. Thromb. Vasc. Biol., DOI: 10.1161/ATVBAHA.110.207142).
When Merck once again set its sights on the clinic, it took baby steps, Mitchel says. The company started with a moderately sized 1,600-patient Phase III trial. The study, called DEFINE, was designed to give researchers “some inkling” of what to expect in terms of anacetrapib’s cardiovascular effects, Mitchel says. DEFINE investigators concluded that there was more than a 90% probability that anacetrapib would not share torcetrapib’s cardiovascular risks. Among patients taking anacetrapib, DEFINE investigators observed a 39.8% decrease in LDL and a 138.1% increase in HDL.

Merck is now enrolling patients in its 30,000-patient REVEAL trial, which Mitchel says is designed to definitively determine whether blocking CETP translates to fewer cardiovascular events such as heart attacks or strokes. “The bottom line is we’re all awaiting the data,” he says, which aren’t expected until 2015 at the earliest.
At Roche, the torcetrapib news spurred additional safety studies for the firm’s CETP inhibitor dalcetrapib, says David Kallend, global medical leader of dalcetrapib. The company launched a pair of Phase IIb safety studies called dal-VESSEL and dal-PLAQUE at about the same time as its 15,600-patient Phase III trial dal-OUTCOMES (Curr. Med. Res. Opin., DOI: 10.1185/03007995.2010.536207). Results from both safety trials emerged last fall. The dal-VESSEL trial showed that dalcetrapib increased levels of HDL by 31% without increasing blood pressure. Researchers conducting dal-PLAQUE used imaging technology to get a snapshot of what was happening to arteries after dalcetrapib treatment. They found no evidence of artery wall abnormalities or side effects among people taking dalcetrapib for two years (Lancet, DOI: 10.1016/S0140-6736(11)61383-4).
Dalcetrapib, which Roche in-licensed from Japan Tobacco Inc. in 2004, is unique among the CETP inhibitors that have reached late-stage trials. Dalcetrapib binds through a transient covalent bond to CETP’s cysteine-13, whereas other inhibitors have only noncovalent interactions with CETP.
Whether the binding mechanism is the key factor that will benefit patients is still to be determined. Clinical-trial results indicate that dalcetrapib raises HDL with a favorable safety profile, and without any of torcetrapib’s side effects, Kallend says. Roche is anticipating results from its pivotal dal-OUTCOMES trial, which is slated to be completed in May 2013, according to the federal clinical-trial database, ClinicalTrials.gov.
Lilly’s lead CETP inhibitor drug candidate, evacetrapib, was still in preclinical development when the torcetrapib news broke, says Nathan B. Mantlo, senior research fellow in discovery chemistry at the company. Lilly was using assays to screen for CETP inhibitors that wouldn’t raise blood pressure as early as 2004, when Pfizer reported the slight elevation in blood pressure from torcetrapib in early-stage trials, he says. And when the torcetrapib postmortem pointed to electrolyte imbalances and high aldosterone levels, Lilly relied on in vitro assays to confirm their compounds didn’t behave similarly (J. Lipid Res., DOI: 10.1194/jlr.M018069).
Advertisement
“We did a comprehensive characterization of evacetrapib’s safety in Phase I clinical trials,” says Holger Schilske, executive medical director of cardiovascular and endocrine research at Lilly. Last fall, Lilly reported results from a Phase II trial of evacetrapib. Investigators were cautious about drawing too many conclusions from the trial, which followed 398 patients. Still, they expressed optimism because in the trial evacetrapib boosted HDL by as much as 129% and lowered LDL by as much as 36% (J. Am. Med. Assoc., DOI: 10.1001/jama.2011.1649). Schilske says investigators found evacetrapib to be well tolerated, with no evidence of adverse effects on blood pressure, aldosterone, or electrolytes.
According to Schilske, a key aspect of the Phase II evacetrapib trial was administering the compound in combination with several statins, including Pfizer’s Lipitor (atorvastatin); AstraZeneca’s Crestor (rosuvastatin); and simvastatin, the generic version of Merck’s Zocor. In the real world, patients are likely to be on a statin if they start taking a CETP inhibitor, so it’s important to monitor patients for drug-drug interactions with evacetrapib, Schilske explains. “We didn’t see any, which is good,” he notes. The company plans to begin Phase III trials of evacetrapib by the end of 2012.
Despite researchers’ diligence to examine what went wrong with torcetrapib, something else could surface in large trials of evacetrapib, anacetrapib, and dalcetrapib that wasn’t detectable in small and midsized trials, Littman cautions. The mechanism of aldosterone elevation in patients taking torcetrapib is still not clear, and multiple factors could have led to the unfavorable outcome in the torcetrapib trial, he says. “There might be something else back there happening behind the curtain.”
The answers to everyone’s CETP questions will have to wait for a few more years, until data from the large Phase III trials arrive, Littman says. “It is still unknown whether blocking CETP will lower cardiovascular event risk,” he says. “That is what it’s all about at the end of the day.”
Still other researchers are wondering whether the basic premise of CETP inhibition—raising levels of HDL, or good cholesterol—is even that good an idea. “I remain unconvinced that HDL modification is going to yield results,” Cedars-Sinai’s Kaul says.
Clinical-trial results with older drugs seem to support Kaul’s position. Clinicians have long prescribed the B-vitamin niacin and a class of amphipathic carboxylic acid drugs called fibrates to patients at risk of heart problems. Among many effects on lipids, these drugs boost HDL levels. But whether the drugs provide long-term health benefits to patients is still not clear. In the Stockholm Ischaemic Heart Disease Secondary Prevention Study, conducted in the 1980s, patients taking niacin and a fibrate had a 36% relative risk reduction in cardiovascular mortality (Acta Med. Scand., DOI: 10.1111/j.0954-6820.1988.tb15891.x).
However, in 2011 the U.S. National Heart, Lung & Blood Institute (NHLBI) stopped the 3,400-person AIM-HIGH study early, after an initial round of data analysis demonstrated that niacin had no benefit on top of statins (N. Engl. J. Med., DOI: 10.1056/NEJMoa1107579). This clinical trial came on the heels of ACCORD, a trial of more than 10,000 patients from which investigators concluded that fenofibrate didn’t give diabetics added benefits on top of a statin (N. Engl. J. Med., DOI: 10.1056/NEJMoa1001282).
Not everyone is convinced the AIM-HIGH and ACCORD trials are the last word on good cholesterol. “I don’t think the door is closed on niacin and fibrates entirely,” says Lee, who has no consulting or financial ties to drugmakers. Drug therapy to raise good cholesterol is in a confusing place today, she says. “Doctors like to have a right and a wrong,” but clinical studies have yet to provide clarity. She says about half of her patients take a fibrate to manage their cholesterol levels.
Merck’s Mitchel notes AIM-HIGH was designed to detect a 25% reduction in heart problem risk at minimum, so if the HDL boost from niacin provided a 10 or 15% risk reduction, it could well go undetected.

An even larger niacin-statin clinical trial than AIM-HIGH, called HPS2-THRIVE, is ongoing. Patients in the trial are receiving a combination of niacin with laropiprant, a molecule designed to cut down on the flushing many patients experience when taking niacin. The Food & Drug Administration rejected Merck’s initial filing for this combination, known as MK-0524A or Cordaptive, in 2008. The agency asked for more safety and efficacy data and suggested waiting for findings from HPS2-THRIVE, which may arrive in 2013.
Even if HPS2-THRIVE provides some clarity on niacin, and even if large clinical trials of CETP inhibitors prove successful, they won’t answer all the outstanding questions about HDL, cautions Kaul, who disclosed a consulting relationship with Roche.
“I’m not quite as convinced as others are that an off-target effect is what led to torcetrapib’s problems,” he says. Researchers have found that HDL plays many roles in the body, including in the immune system and in inflammatory response (Immunol. Cell Biol., DOI: 10.1038/icb.2009.112). Kaul, who sits on FDA panels that judge cardiovascular disease drug candidates, notes that in the ILLUMINATE trial, nine patients on torcetrapib died from infections, whereas none in the control group did (N. Engl. J. Med., DOI: 10.1056/NEJMoa0706628). And 24 patients on torcetrapib died of cancer, compared with 14 in the control group. Any links to infection or cancer are pure speculation at this point, he says, but the data “make you feel at least uncomfortable.”
Perhaps most important for the HDL question is that niacin, fibrates, and all the CETP inhibitors thus far also affect other lipoproteins, such as LDL. Clinical trials of those agents are not a direct test of whether raising HDL prevents cardiovascular disease, Kaul says. The best information available on HDL is epidemiological data and circumstantial evidence, he says. Without a purely HDL-raising drug candidate to test, Kaul says, “the jury’s still out.”






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter