Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
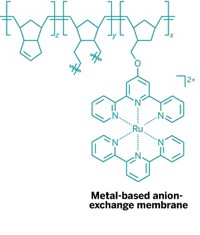
Ionic Membranes Go Metallic
Polymer Science: Cross-linked metal ionomers expand options for fuel-cell applications
by Stephen K. Ritter
March 14, 2012
Incorporating a ruthenium complex into the backbone of a cross-linked polymer has led to the first metal-based anion-exchange membranes. The new materials could make fuel cells more efficient, less expensive, and allow them to operate under a wider range of reaction conditions (J. Am. Chem. Soc., DOI: 10.1021/ja211365r).
A team led by Gregory N. Tew of the University of Massachusetts, Amherst, and Michael A. Hickner of Pennsylvania State University began by building a cross-linkable monomer from terpyridine and ruthenium(II)-bearing norbornene. With ring-opening metathesis, they polymerized the monomer’s norbornene unit, using different amounts of dicyclopentadiene as a cross-linkable comonomer to form a series of anion-exchange membrane thin films.
The new materials demonstrate anion conductivity and mechanical properties on par with existing nonmetal anion-exchange membranes used for applications such as water purification and fuel cells. But they have key advantages over their nonmetal analogs.
One advantage of the ruthenium-based membranes is that the divalent metal carries two anions, Tew says. That capability increases ion-exchange capacity and thus efficiency, compared with monovalent anion materials. “The metal-cation concept should allow us to increase the anion capacity beyond two anions per metal using other, perhaps cheaper, metals,” he adds.
Another benefit of the ruthenium complex is its stability at the high pH and moderate temperature (80 °C) found in alkaline fuel cells, Tew notes. The anion-exchange membrane is typically the most expensive part of an alkaline fuel cell, an application for which the stability of standard quaternary ammonium-based anion membranes has been a concern.
The metal-cation films are “a creative breakthrough in membrane science,” says Ian Manners of the University of Bristol, in England, whose group makes metallopolymers.
Anion-exchange membranes have been dominated by polymeric materials based on quaternary ammonium groups, Manners explains. On the other hand, metal-cation-containing polymers are well-established, and they have found applications as phosphorescent emitters and sensors and in redox-active gels, he adds. But putting the two concepts together “is a significant new development,” Manners says.
The new polymer design has been patented, Tew says, but the team hasn’t yet moved forward with any commercialization efforts.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter