Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Cancer-Killing Antibody Does Double Duty
Drug Development: Antibodies that bind two molecules can deliver cancer cells to immune cells for annihilation
by Erika Gebel
June 6, 2012

Antibody drugs can bind specific biomolecules to treat illnesses including cancer and autoimmune diseases. Each antibody typically targets only one molecule. Now researchers have developed a simple method to make stable bispecific antibodies, which can bind two molecules (J. Am Chem. Soc., DOI: 10.1021/ja303904e). They say the antibodies offer novel ways to fight disease.
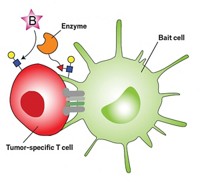
Researchers have made other bispecific antibodies but almost all of the ones that drug companies are working on are fusion proteins, says Vaughn Smider of Scripps Research Institute, in La Jolla, Calif. Fusion proteins, which are made by stitching two antibody genes together to encode a single protein chain, tend to be unstable, says Smider. Their instability makes them difficult to develop into medications. Smider hoped to make a more stable molecule by producing the two halves of a bispecific antibody separately and then joining them together chemically.
For the two halves, Smider and his colleagues selected one antibody fragment that binds a molecule on breast cancer cells and another that sticks to proteins on immune cells called killer T cells. Their idea was that the bispecific antibody could bring the two cells into close proximity, allowing the T cells to kill cancer cells. The researchers incorporated an unnatural amino acid, p-acetylphenylalanine, into each fragment’s sequence as an anchor to attach an ethylene glycol linker. When the researchers mixed the two fragments together, reactive groups on the ends of the linkers united the fragments into a single bispecific antibody.
To test the construct, the researchers added it to a culture of breast cancer and T cells. The bispecific antibody killed about half of the cancer cells at a concentration of 20 pM. Uncoupled fragments did not kill cancer cells, nor did the bispecific antibodies kill cells that lacked the breast cancer antigen. Smider next plans to make a bispecific antibody that kills prostate cancer cells and to test the molecules in animal experiments.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter