Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Evaluating Enzyme Inhibitors
Drug Development: Assay allows researchers to rate reversible enzyme inhibitors in animal experiments
by Laura Cassiday
June 13, 2012

Small-molecule inhibitors of enzymes can, in principle, treat human disease. But first, researchers must confirm that an inhibitor actually binds its enzyme target in animal experiments. This can be a difficult task for poorly characterized enzymes that lack easily measurable substrates and products. Now researchers have found a new way to measure how well an inhibitor binds its target enzyme in mice (J. Am. Chem. Soc., DOI: 10.1021/ja303400u).
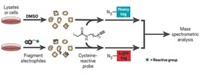
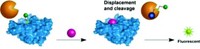
The assay is based on one that Benjamin Cravatt, a chemical biologist at Scripps Research Institute, La Jolla, Calif., and his colleagues previously developed (Annu. Rev. Biochem., DOI: 10.1146/annurev.biochem.75.101304.124125). In that assay, a fluorescent probe makes a covalent bond to an enzyme’s active site. The researchers inject mice with an inhibitor, followed a few hours later by the probe. After another hour, the scientists sacrifice the animals, extract protein from their tissues, and examine the fluorescent signal of the target enzyme. The more strongly an inhibitor binds to an enzyme, the less likely it is that the probe will bind and, as a result, the less fluorescence the enzyme will show.
However, the assay only works with so-called irreversible inhibitors, which form covalent bonds with the enzyme. Because the probe reacts quickly and irreversibly with the enzyme, reversible inhibitors, which interact only fleetingly with enzymes, simply can’t compete. So Cravatt and his coworkers identified a slower-reacting probe to level the playing field. The researchers had developed the probe, a triazole urea compound, in a previous study (Nat. Chem. Biol., DOI: 10.1038/nchembio.579), and determined in this study that it reacted slowly with the enzyme.
The team used the new probe to gauge reversible inhibitors for the enzymes LYPLA1 and LYPLA2, which influence cell signaling by removing certain chemical groups from proteins. They found that the best reversible inhibitors had a feature that previously identified irreversible inhibitors did not: Each inhibitor interacted with only one of the two related enzymes.
Cravatt says that the new method should allow researchers to better characterize LYPLA1 and LYPLA2, which so far have lacked selective inhibitors that work in animal experiments. He thinks that the assay could easily work with other enzymes, assuming researchers can find a slow-reacting probe.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter