Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Greenhouse Gases
New Clues For Capturing CO2
Carbon Scrubbers: To help battle climate change, researchers make metal-doped materials that can remove carbon dioxide directly from air
by Olga Kuchment
June 28, 2012

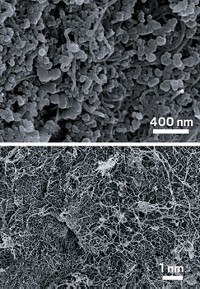
A sprinkling of zirconium helps a carbon dioxide scrubber material capture up to four times as much CO2, researchers have found (J. Am. Chem. Soc., DOI: 10.1021/ja303136e). The addition also makes the material more resilient to repeated use.
To reduce carbon emissions, scientists and engineers are searching for solid materials that can grab CO2 directly from air. These materials could potentially generate CO2 for use in chemical and polymer production or for underground sequestration. So far, the search has focused on varying the design of amines encased in porous silica. Attempts to vary the supporting material have been rare, says Christopher W. Jones, a chemical engineer at Georgia Institute of Technology.
Jones thought he could improve CO2 binding by embedding metal atoms in silica along with an amine. He reasoned that these atoms could activate the amines by changing their access to, or affinity for, CO2. Other researchers had used transition-metal silicates to catalyze reactions that use CO2.
Modifying slightly the standard methods for creating the carbon-scrubbing materials, Jones and his collaborators mixed various metals into silica and then added polyethylenimine. They tested how well the scrubber materials worked by weighing them in a flowing gas, either 10% CO2 in argon to simulate the flue gas from a power plant, or 400 parts per million CO2 in argon to simulate ambient air. The materials that picked up the most weight, the researchers reasoned, had adsorbed the most CO2.
After screening titanium, aluminum, and cerium, the team saw efficiency increase dramatically with zirconium. Seven percent zirconium worked best. At higher percentages, the structure of the silicate appeared to degrade, making the scrubber less effective.
Compared with undoped silica the 7% zirconium material adsorbed twice as much CO2 from the flue-gas setup and four times as much when the CO2 was diluted to 400 ppm. The team stripped the CO2 from the scrubber material by heating it in flowing argon, readying it for reuse. The material’s capacity for CO2 adsorption decreased only slightly after four uses.
Chunshan Song, a chemical engineer and fuel scientist at Pennsylvania State University, says the increase is consistent with, but much greater in magnitude than, what his group saw when doping silica with aluminum to use as the amines’ support.
Abdelhamid Sayari, a chemist at the University of Ottawa, calls the increase “dramatic” and says he would like Jones to explore how the metal creates the increase in adsorption and to develop even more powerful adsorbents.
Jones’s team plans to do exactly what Sayari suggests. Jones also collaborates with a company, Global Thermostat, to develop commercial processes for extracting CO2 from air.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter