Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
A Hydrogel That’s Soft, Stretchy, And Tough
Materials Science: Soft material has unusual properties, thanks to a blend of ionically and covalently cross-linked polymers
by Bethany Halford
September 6, 2012

A hydrogel with record-busting toughness that’s superstretchy to boot has been created by a team of engineers (Nature, DOI: 10.1038/nature11409). The new material could improve the performance of products made from hydrogels, such as flexible contact lenses and tissue engineering scaffolds. It could also open up applications for which hydrogels were previously considered too prone to breakage to be useful, such as in materials for cartilage replacement.

\

\
Alginate-polyacrylamide hydrogel stretches 20 times its original length without breaking.
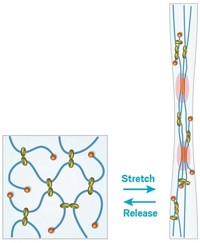
Hydrogels are made mostly of water—roughly 90%—held into a solid form by water-insoluble polymers. They don’t typically stretch very far, and they are usually quite brittle, with a fracture energy of only about 10 J/m2. By contrast, the new hydrogel has a fracture energy of about 9,000 J/m2 and can stretch to 20 times its initial length without breaking.
The real breakthrough in creating tougher, stretchier hydrogels came in 2003, says Zhigang Suo, the Harvard University mechanical engineering professor who led the development of the new hydrogel. That’s when a team led by Jian Ping Gong, a professor at Hokkaido University, in Japan, came up with the idea of covalently linking two types of polymers in a hydrogel (Adv. Mater., DOI: 10.1002/adma.200304907).
These so-called double-network hydrogels feature a primary polymer network that’s dense in covalent cross-links and a secondary polymer network with fewer cross-links. The primary network is quite brittle and will fracture under significant strain, breaking its covalent cross-links. When that happens, the secondary network disperses the energy through the rest of the hydrogel, lending the material toughness.
The problem with Gong’s hydrogels was that all the polymers were covalently crosslinked, so they could take stress after, but once those bonds were broken they didn’t re-form and the material failed under repeated stress.
Suo’s team came up with the idea of using alginate, a polymer that forms ionic cross-links in the presence of calcium ions, as the primary network. The alginate will break at these ionic cross-links under stress, but the cross-links will re-form when the stress is relieved.
Combining alginate with covalently cross-linked polyacrylamide in a 1:8 ratio creates a material that’s far tougher than either material on its own, Suo notes. Furthermore, if the hydrogel is pierced, that hole won’t propagate through the material, even when it is stretched to 17 times its original length. So, Suo says, one could imagine stitching through the hydrogel without making it prone to breakage when it’s stretched.
The synthesis of the hydrogel is simple, Suo says. “We’re mechanical engineers. We don’t know much chemistry,” he confesses. The researchers essentially just mix two powders in water and shine ultraviolet light on the solution. “There are no fancy materials,” Suo says. “Labs around the world can try it in a matter of weeks.”
“It is amazing to see that the double-network structure can work out so excellently,” comments Hokkaido University’s Gong. “This work will encourage polymer scientists to pursue more possibilities for hydrogels that still have few practical applications at the moment,” she says.
“The depth of the mechanics analysis is what makes this research particularly impressive,” adds Kenneth R. Shull, a materials science professor at Northwestern University. The researchers not only provide detailed answers about the properties soft materials can possess, he says, but they also leave soft-matter scientists with some interesting questions too, such as how these materials behave on a molecular level.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter