Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Lewis Acid-Base Pair Assists Enzyme Mimic
Catalyst ligand’s design emulates the secondary-level binding of a metalloenzyme’s active site
by Stephen K. Ritter
March 11, 2013
| A version of this story appeared in
Volume 91, Issue 10
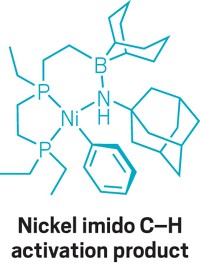
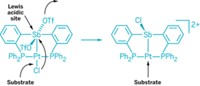
In the search for better catalysts, chemists often try to emulate an enzyme’s ability to efficiently bind, activate, and functionalize a reactant molecule. In the latest twist, a University of Michigan research team has designed a transition-metal complex featuring a ligand with functional appendages that mimic the secondary coordination sphere of a metalloenzyme’s binding pocket. Oscar Tutusaus, Chengbao Ni, and Nathaniel K. Szymczak synthesized a vanadium terpyridine complex with one pyridine ring bearing a boronic ester Lewis acid and another pyridine ring bearing a morpholine Lewis base (J. Am. Chem. Soc., DOI: 10.1021/ja400962h). Their design is reminiscent of so-called frustrated Lewis pair systems, which have pent-up catalytic reactivity because of the inability of the acid-base pair, held apart by bulky substituents, to completely combine to form a neutral adduct. The Michigan researchers tested their new complex through its reaction with hydrazine (N2H4). They found that the nitrogen atom of the Lewis base and the boron atom of the Lewis acid help hold hydrazine in place for vanadium to pull apart the N–N bond (shown), with the Lewis pair acting in the same way that peripheral amino acids in an enzyme’s binding site help hold a substrate. Chemically reducing vanadium slices the N–N bond to form ammonia, a key action of nitrogenase enzymes and an important intermediate step in the industrial process for making ammonium-based fertilizers. The synergistic transition-metal/Lewis pair interaction could become a general methodology for developing new catalysts, Szymczak says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter