Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Getting To Alzheimer’s Early
Biomarkers and imaging tools may show whether new therapies stop damage before it’s too late
by Lauren K. Wolf
March 18, 2013
| A version of this story appeared in
Volume 91, Issue 11

One of the most debilitating, fatal diseases the world has known got its first mention in 1906 during a run-of-the-mill research conference in Tübingen, Germany. There, psychiatrist Alois Alzheimer described his interactions with a 51-year-old asylum patient named Auguste Deter.
The woman, Alzheimer explained to his peers, had severe memory impairments, was frequently disoriented, and at times thought someone was trying to kill her. Just before the conference, Deter succumbed to her illness, so the German psychiatrist also described what he found during an autopsy of her brain.
Silver stains, he said, revealed fibrils of unique thickness as well as seedlike lesions distributed throughout Deter’s brain. “It seems we are dealing here with a special illness,” Alzheimer wrote in a paper published the following year (Clin. Anat., DOI: 10.1002/ca.980080612).
Today, the only way to definitively diagnose a patient with Deter’s illness—dubbed Alzheimer’s disease, after its discoverer—is still a brain autopsy. And testing potential treatments in a dead body is, to say the least, a challenge.
In the past decade, however, scientists have developed brain imaging techniques and biomarker assays for diagnosing Alzheimer’s during life rather than after death. Although most of them aren’t yet accurate enough for routine screening of patients, these tools are changing the face of Alzheimer’s disease research.
Not only are they being used to uncover the progressive molecular and cellular changes caused by the memory-robbing disease, they’re also helping researchers select the optimal patients for treatment with experimental therapies. During a trio of early-intervention trials set to start up this year, the techniques will monitor how well drug candidates hit their targets.
Alzheimer’s today afflicts about 5 million people in the U.S., at least 96% of whom are age 65 and older, according to the Alzheimer’s Association. Scientists hope that, with further validation, biomarker assays and imaging tools might one day be combined with successful therapeutics to prevent the disease.
If a drug does eventually come to light that staves off Alzheimer’s, “you might imagine a future where, in addition to your preventive colonoscopy every five years after you reach middle age, you would get your Alzheimer’s imaging and biomarker exam too,” says John Q. Trojanowski, a neuropathologist at the University of Pennsylvania’s Perelman School of Medicine. “If you’re positive, you would get treated.”

In the meantime, early diagnosis could help present-day patients. Alzheimer’s is just one form of dementia, a loss of mental function caused by the death of nerve cells in the brain. Prior to a patient’s death, a physician might suggest a “probable” diagnosis of Alzheimer’s on the basis of cognitive and other tests. But an Alzheimer’s diagnosis is certain only when pathologists peek inside the brain at autopsy and locate the telltale fibrils composed of tau protein and seedlike plaques of amyloid peptides.
“People are symptomatic for years before they get a formal diagnosis of dementia,” says Christopher C. Rowe, a behavioral neurologist at the University of Melbourne, in Australia. During this intermediate stage—called mild cognitive impairment (MCI)—patients undergo repeated tests, and they and their families have to live with the uncertainty of their condition.
Only 50% of people with MCI go on to develop Alzheimer’s, explains Rowe, who is also director of nuclear medicine at Melbourne’s Austin Hospital. Earlier diagnosis could give these patients peace of mind, he contends. It also could help those predicted to get the disease make financial and living arrangements themselves, before they’ve lost the capacity to do so.
Recognition that molecular changes in the brain initiate well before the onset of dementia began in the 1990s, when MCI was identified. During that decade, scientists began extracting from patients a liquid that surrounds the spinal cord and brain to examine its contents.
Some of the molecules they found in this cerebrospinal fluid (CSF)—amyloid peptide fragments and versions of tau protein—changed in concentration as MCI progressed toward full-blown dementia. So the researchers postulated that molecular CSF levels might be good predictors, or biomarkers, of disease evolution.
But scientists quickly became mired in efforts to establish absolute CSF values for predicting disease. The community realized that labs were using different protocols to collect and analyze the liquid and thus getting disparate results, Trojanowski says.
So a group of researchers, including the UPenn neuropathologist, banded together to standardize CSF methods and to develop other early diagnosis techniques, such as imaging. The scientists launched the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a public-private partnership, in 2004.
Since then, the group has tested more than 1,000 elderly subjects, some with probable Alzheimer’s, some with MCI, and some who are cognitively normal. During the course of their studies, ADNI researchers have learned that concentrations of overall tau protein and of a phosphorylated version of tau, p-tau(181), increase in CSF as people progress toward dementia. At the same time, the level of a particular peptide fragment, amyloid-β42, decreases in CSF as it gets sequestered by brain plaques.
Even though ADNI and other biomarker programs have nailed down these trends with certainty, no one has gotten a handle on the absolute CSF biomarker levels that will predict Alzheimer’s.
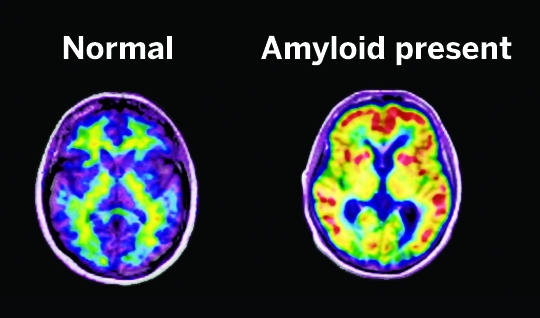
\

\
Radiotracers such as Pittsburgh Compound B (red indicates high concentration) and Amyvid stick to amyloid in the brain and give away their presence on PET scans.
To get a firmer grip, an international research team, funded by the National Institutes of Health and the Alzheimer’s Association, is assessing commercial antibody-based assays for detecting Alzheimer’s biomarkers in CSF. In a 2011 experiment, a neurochemistry lab in Sweden mailed the same CSF samples to 40 labs around the globe for analysis. Absolute values reported back for three common Alzheimer’s biomarkers varied by 13 to 36% (Alzheimer’s Dementia, DOI:10.1016/j.jalz.2011.05.2243).
In hopes of bringing the variance down, scientists are now taking a closer look at CSF protocols. “The devil’s in the details,” says Anne M. Fagan, a neuroscientist at Washington University in St. Louis. For instance, she says, researchers have so far learned that amyloid-β42 levels in CSF fluctuate in a regular daily pattern. “That’s why we collect CSF from individuals at the same time of day,” she says of her lab.
“Maybe there will be a gold standard CSF assay kit for Alzheimer’s someday, but we’re not there yet,” Fagan says.
Even better than CSF biomarkers for diagnosing Alzheimer’s, though, might be blood biomarkers, experts say. After all, patients would undoubtedly prefer the drawing of blood to the lumbar puncture required to get at CSF. “But there are a million things you can measure in blood,” Austin Hospital’s Rowe says. So progress on developing a viable Alzheimer’s blood test has been slow.
Over the past eight years, ADNI has, however, made major advances in imaging tools. Researchers have shown that magnetic resonance imaging (MRI) can track early shrinkage of the brain that corresponds with Alzheimer’s development. And positron emission tomography (PET) can now map key buildups in the brain.
In PET, “reporter” molecules, called radiotracers, are injected into patients, cross the blood-brain barrier, and latch onto target molecules there. Scientists are able to pinpoint the tracers’ locations with a PET scanner because each molecule contains a radioactive positron-emitting isotope.
When designed to bind to amyloid or tau in the brain, these compounds might track a person’s progression toward Alzheimer’s. At the moment, tau radiotracers are still in early trials, but amyloid reporters are in regular use.
The first successful radiotracer to stick to amyloid plaques was developed and then tested by geriatric psychiatrist William E. Klunk and radiologist Chester A. Mathis at the University of Pittsburgh in the early 2000s. Called Pittsburgh Compound B, the molecule has been mostly restricted to use in academic labs because it contains a 11C isotope with a 20-minute half-life. That means its signal decays by 50% every 20 minutes, so it has to be synthesized on-site.
To overcome this limitation, chemists have developed radiotracers with longer-lasting 18F isotopes. One of these amyloid-binding compounds, Eli Lilly & Co.’s Amyvid, was approved last year by the Food & Drug Administration. With a half-life of two hours, Amyvid can be generated by a cyclotron at a regional center and shipped same-day to hospitals running PET scans, says Daniel M. Skovronsky, chief executive officer of Avid Radiopharmaceuticals.
Avid, which Lilly purchased in 2010, demonstrated the efficacy of its product for FDA by administering it to about 60 patients near the end of their lives and running PET scans. Once the patients died, the firm compared the presence of amyloid at autopsy with PET scan readings by five radiology experts. For patients who died within a year of having a PET scan, Amyvid correctly signaled the presence of amyloid 96% of the time (Lancet Neurol., DOI: 10.1016/s1474-4422(12)70142-4).
One limitation of Amyvid right now is that the radiotracer doesn’t come cheap. The price for the drug, a PET scan, and a radiologist’s time is about $3,000, a cost that Medicare isn’t yet covering.
And at this point, Amyvid PET scans can only rule out Alzheimer’s for patients with symptoms of cognitive impairment, Skovronsky says. They can’t be used to diagnose a person with the disease.
According to experts in the community, that’s because pathologists find amyloid plaques in the brains of about 30% of cognitively normal people who die late in life. The mere presence of amyloid doesn’t always mean a person has or will get Alzheimer’s.
One way to interpret the phenomenon, Washington University’s Fagan proposes, is to assume these people possess a protective molecular mechanism that holds at bay the nerve cell damage normally wrought by Alzheimer’s. Another, she adds, is to assume that if those people had lived long enough, they would’ve eventually yielded to the disease.

This debate might get settled in the next five to six years during the Antiamyloid Treatment in Asymptomatic Alzheimer’s Disease clinical trial, nicknamed A4. Researchers co-led by neurologist Reisa A. Sperling of Brigham & Women’s Hospital, in Boston, will select 1,000 participants for the study, beginning in October, by running Amyvid PET scans on their brains. If a person is PET-positive for amyloid, is at least 70 years old, and is mentally normal at the start of the trial, he or she will be included.
Half the participants will eventually receive a monoclonal antibody drug, Lilly’s solanezumab, which binds to amyloid-β monomers and, scientists believe, clears them from the brain. The other half of the group will get a placebo. They’ll all be monitored periodically by PET and MRI scans, CSF assays, and cognitive tests.
But it’s the placebo cohort that might be able to give researchers answers about how fast people with amyloid in their brains develop Alzheimer’s, if ever.
Two research groups last year reported what might be one piece of the Alzheimer’s timeline puzzle. Using amyloid PET imaging and CSF assays, the teams studied patients with genetic mutations that guarantee Alzheimer’s onset early in life. They found that amyloid begins accumulating in the brain 15 to 25 years before mental function begins to decline (N. Engl. J. Med., DOI: 10.1056/nejmoa1202753; Lancet Neurol., DOI: 10.1016/s1474-4422(12)70227-2).
Because biomarker assays and imaging studies have all indicated that amyloid accumulation in the brain is always the first step along the Alzheimer’s pathway, many scientists think that when the peptide aggregates, it somehow triggers tau protein to do the same. In their view, tau then forms fibrillary tangles inside nerve cells that ultimately cause damage and memory loss. Changes in tau, researchers say, track more closely with the appearance of cognitive symptoms, whereas amyloid plaque seems to stop accumulating much earlier.
Recent Phase III clinical trial failures of drugs designed to clear amyloid from the brain, however, have some experts questioning whether this “amyloid cascade hypothesis” is valid. Pfizer’s bapineuzumab failed to improve mental function in patients with mild to moderate Alzheimer’s last year. And Lilly’s solanezumab had mostly negative results.
Advertisement
According to William Jagust, a neurologist at the University of California, Berkeley, “A person could draw two conclusions from these failed trials.” One is that amyloid is the wrong target for treating Alzheimer’s, and the other is that the drugs were administered too late to have an effect. Both drugs were given to patients who were diagnosed with probable Alzheimer’s and thus already had significant, irreversible nerve cell damage.
Researchers involved in three new trials—including the A4 study—are hoping the drugs were just too late. They will give various antiamyloid therapies to cognitively normal subjects who are genetically predisposed to get Alzheimer’s or, in the case of A4, who have been confirmed to have amyloid in their brains.
One of these trials, set to begin initial patient visits this month, is being conducted by the Dominantly Inherited Alzheimer Network (DIAN), a collection of international researchers studying people with genetic mutations that lead to Alzheimer’s. Genetic versions of the disease, called familial Alzheimer’s, are passed from parent to child and set in early in life, between the ages of 30 and 60. DIAN will follow 240 subjects with or without some of the gene abnormalities that lead to Alzheimer’s.
Researchers will also give subsets of DIAN patients one of three amyloid-targeted drugs.
Another of the early-intervention trials, run by the Alzheimer’s Prevention Initiative (API), will follow patients who share a single specific mutation in a gene that codes for the presenilin-1 protein. They are members of a large extended family, many of whom live in and around Medellin, Colombia. Of the 5,000 kindred, 30% have the presenilin mutation that causes cognitive impairment by age 45.
“We’re excited about the chance to give these family members, who are at the highest imminent risk of Alzheimer’s, access to an investigational treatment and empower them in this fight against the disease,” says Eric M. Reiman, executive director of Banner Alzheimer’s Institute and one of the leaders of the API study. One-half of the API trial participants will receive the monoclonal antibody crenezumab, an amyloid-clearing drug from Genentech.
According to Reiman, the DIAN, API, and A4 trials should provide the best test yet of the amyloid cascade hypothesis. That’s because they’ll be able to evaluate antiamyloid treatments at a very early stage in Alzheimer’s. And scientists will be able to track whether the drugs have an effect with imaging and biomarker tools.
“But what we really want to know,” the A4 trial’s Sperling says, “is if you lower amyloid burden, do you make the brain work better?” So at the same time, the clinical trials will also test whether the drugs hold mental decline at bay.
“If the treatments don’t work,” Reiman adds, “these studies should encourage the field to discover therapies that target elements of the disease other than amyloid.”
In the past five to 10 years, Austin Hospital’s Rowe says, “there’ve been such great advances in Alzheimer’s diagnosis that we’ve now moved ahead of therapeutics.” Drug development needs to come up to speed, he argues, “to really make these tools important to patients in the community.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter