Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Sulfide Signal In Sight
Chemical Biology: Probe tracks molecule that can be toxic or beneficial
by Carmen Drahl
April 22, 2013
| A version of this story appeared in
Volume 91, Issue 16
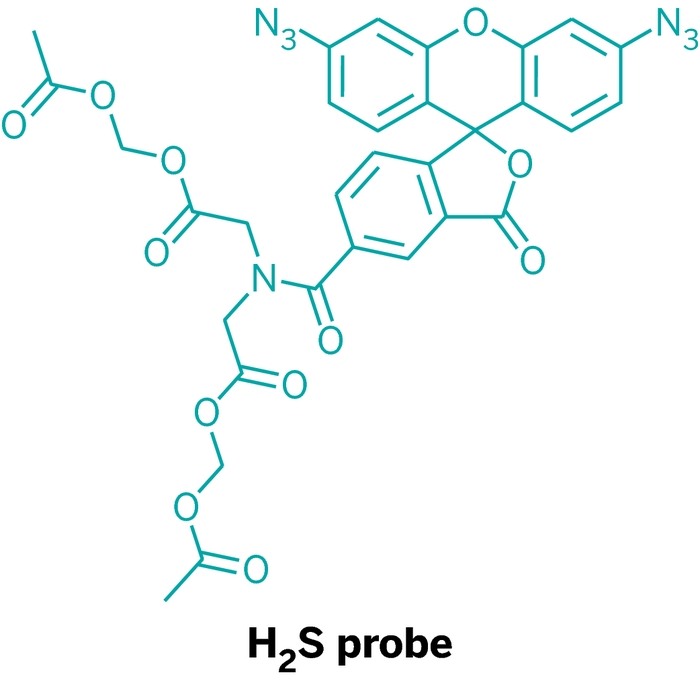
\
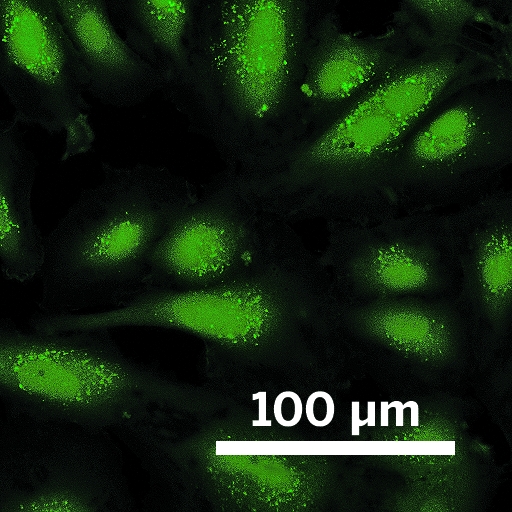
\
In this confocal microscopy image, live human umbilical cord cells glow green as Chang’s probe detects H2S inside them.
Infamous for being the source of rotten-egg stench, hydrogen sulfide (H2S) has a rather complicated place in biology. The human body makes it. It is both poisonous—linked to diseases such as type 1 diabetes—and beneficial to activities such as immune signaling. Researchers would like to know more about how H2S is made and how it exerts its effects, but they have lacked tools to noninvasively monitor the compound in living beings.
Enter Christopher J. Chang’s team at the University of California, Berkeley, which has developed molecules to track H2S in live cells (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1302193110).
Chang’s lab first reported fluorescent probes for H2S two years ago. The probes selectively light up in the presence of H2S because they contain an aryl azide group that only H2S can reduce to a fluorescent product. Those probes, however, weren’t sensitive enough to detect biologically relevant levels of H2S.
To fix that, Chang lab members Vivian S. Lin and Alexander R. Lippert made two improvements. They increased the probes’ sensitivity by adding a second aryl azide group. They also added two acetoxymethyl ester groups, which are lipophilic and help the probe enter cells. Once inside, the groups get cleaved by esterases, rendering the probe incapable of leaving. The probes accumulate inside cells, further boosting sensitivity.
With the improved probes, the team followed H2S production in cells from umbilical cords. They found evidence to back a hunch held by members of several other labs—H2S production depends on a signal from hydrogen peroxide. That’s a fascinating relationship, Chang says, because H2O2 is an oxidant and H2S is a reductant. The biochemistry linking those chemical opposites is far from clear, so “it’s going to be exciting to learn more about that cross talk,” he says.
“This work presages many exciting new directions in both redox and chemical biology,” says Kate Carroll, who maps redox signaling at Scripps Research Institute Florida. “A central goal for future studies will be to define the molecular targets of H2O2, H2S, and other redox-based signaling molecules.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter