Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Thin Inorganic Ribbons Coil And Flex
Ability to bend imparts biomolecule-like self-assembly qualities to ordinarily rigid inorganic structures
by Mitch Jacoby
May 6, 2013
| A version of this story appeared in
Volume 91, Issue 18
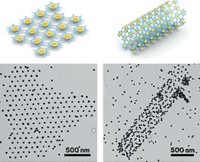
If inorganic compounds could learn a trick or two about flexibility from biomolecules, they could be transformed into versatile building blocks for complex structures. Proteins and other long biomolecules gracefully bend, flex, and fold. That agility enables those compounds to assemble into a large variety of complex ordered structures. By contrast, inorganic wires, rods, and other elongated structures tend to be rigid, which limits the complexity and variety of structures they form. Researchers in China have now shown that indium sulfide, when prepared in ultrathin nanoribbon form, flexes and assembles in biomolecule-like ways. Tsinghua University chemists Peng-peng Wang, Xun Wang, and coworkers report that long InS ribbons just 9 Å thick are flexible enough to spontaneously coil into a variety of shapes including double-headed and S-shaped coils (J. Am. Chem. Soc., DOI: 10.1021/ja403065z). They also find that in response to the presence of alkylamines and other synthesis conditions, the coils adjust their shapes and spontaneously assemble into a variety of two- and three-dimensional ordered superlattices. Ultrathin nanocoils may lead to new insights into using flexible inorganic nanocrystals as building blocks for superstructures, the group predicts.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter