Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Alloy Makes Iron On The Cheap
Chromium-iron electrode smelts iron without the carbon dioxide
by Craig Bettenhausen
May 13, 2013
| A version of this story appeared in
Volume 91, Issue 19
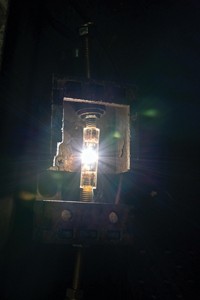
Electrodes made of a chromium-iron alloy may reduce the energy consumption and carbon footprint of iron production. Conventional smelters use carbon (coke) to chemically reduce ores composed of iron oxides. The process releases that carbon in the form of CO2, emitting a massive quantity of the greenhouse gas into the atmosphere. Now, Donald R. Sadoway, Lan Yin, and Antoine Allanore of Massachusetts Institute of Technology have directly separated the iron and oxygen in iron ore in a bench-scale electrolysis experiment by using an anode made of a cheap alloy of the Earth-abundant metals chromium and iron (Nature 2013, DOI: 10.1038/nature12134). Other groups have demonstrated similar metal oxide electrolysis with iridium electrodes, but that rare element is too expensive for wide use. The MIT team’s alloy anode produces higher purity iron than that made by conventional smelting methods and liberates molecular oxygen as a secondary product. The iron-smelting industry, which has a capacity of billions of metric tons per year, could reap efficiency gains from processes based on the alloy, says University of Cambridge materials chemistry professor Derek Fray in a commentary about the work. The process could also provide breathable oxygen to astronauts in space, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter