Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Solving An Old Bonding Debate
Crystallography: 2-Norbornyl cation structure shows nonclassical bonding at last
by Jyllian Kemsley
July 8, 2013
| A version of this story appeared in
Volume 91, Issue 27

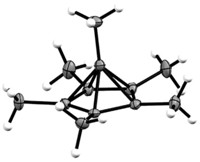
Chemists have used a deep-freeze crystallization method to solve the structure for the 2-norbornyl cation, likely closing an acrimonious 50-year debate on the nature of a fundamental bonding concept. Instead of two electrons shared between two carbon atoms, as in conventional single-bond structures, this cation structure shows nonclassical bonding, with two electrons delocalized over three carbon atoms (Science 2013, DOI: 10.1126/science.1238849).
The structural analysis of the cation, a bridged cyclic hydrocarbon, was led by Ingo Krossing of the University of Freiburg and Karsten Meyer of the University of Erlangen-Nuremberg, both in Germany.
The debate centered on different interpretations of thermodynamic and spectroscopic data for the cation. Last year, University of Richmond chemist and historian Jeffrey I. Seeman characterized the interactions between the two sides as “crude and rude” (C&EN, Nov. 19, 2012, page 44). A crystal structure of the cation likely would have resolved the arguments, but that experimental evidence was elusive. Many other researchers have obtained 2-norbornyl cation crystals, but their molecular disorder stumped scientists, Krossing says.
Meyer, Krossing, and colleagues managed to obtain the structure by stabilizing the 2-norbornyl cation with a bromoaluminate counterion, crystallizing the product for several days at a chilly 245 K. They developed an annealing process of cooling the crystals even further to 40 K, then repeatedly warming and cooling the crystals to freeze out molecular disorder, allowing them to collect good X-ray data.
The structure reveals the two-electron, three-carbon system as having two C–C bonds of 1.80 Å, longer than a standard 1.54-Å C–C single bond. The third bond in the system is 1.39 Å long, similar to the delocalized C–C bonds of benzene.
The work is “a triumph of creative crystallography in taming an uncooperative cation,” says theoretical organic chemist Dean J. Tantillo of the University of California, Davis. Although the existing evidence had largely convinced chemists that the nonclassical structure existed in the gas phase and in solution, the new structure demonstrates that it exists in the solid state as well, Tantillo says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter