Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Chemical Treatment Alleviates Nanotube Toxicity
Safety: Hydrophilic modifications render asbestos-like nanotube bundles harmless in mice
by Bethany Halford
January 17, 2013
| A version of this story appeared in
Volume 91, Issue 3
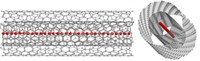
Chemically modifying the surface of certain carbon nanotubes can reduce their asbestos-like toxicity, scientists report (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201207664). The finding could lead to safer nanotube-based products.
Nanoscientists were alarmed to learn in 2008 that multiwalled carbon nanotube fibers that are at least 20 μm in length trigger a toxic response in mice akin to what asbestos does. “The report scared the entire scientific community and still prompts skeptical attitudes whenever we talk about nanotubes for biological applications,” says Maurizio Prato, a nanotube expert at Italy’s University of Trieste.
So Prato, along with Alberto Bianco of France’s National Center for Scientific Research and Kostas Kostarelos of University College London, set out to see whether potentially dangerous nanotubes could be transformed into safer material. They took the same nanotubes that researchers had previously shown to be dangerous and then covalently modified their surfaces.
To one group of nanotubes they added long alkyl chains and to another they added hydrophilic chains containing oxygen atoms and an amino group on their ends. In mice, the tubes with alkyl chains had the same type of asbestos-like toxicity as pristine nanotube fibers. The nanotubes with hydrophilic chains, however, appeared to be harmless.
“This type of chemical functionalization reaction led to better dispersion and broke bundled nanotubes apart, thereby reducing their effective length so they did not prompt a reaction like asbestos fibers,” Bianco explains.
Jun Kanno, a researcher at Japan’s National Institute of Health Sciences who led one of the 2008 studies, is skeptical of the group’s conclusions. He wonders whether the chemical functionalization process skims off the outer layers of the nanotubes and introduces structural defects that make them thinner and shorter, and therefore less toxic.
Prato and coworkers say only the surface of the nanotube is functionalized and that the dispersion of nanotube bundles is what reduces toxicity.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter