Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
B Family Album
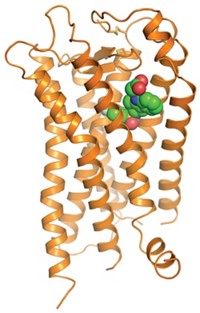
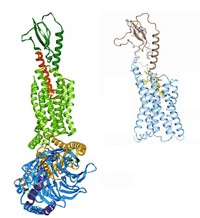
Structural Biology: First structures of class B G protein-coupled receptors may aid drug hunts
by Carmen Drahl
July 25, 2013
| A version of this story appeared in
Volume 91, Issue 30

As many as 30% of drugs on the market target G protein-coupled receptors (GPCRs), a family of signaling conduits that snake back and forth across cell membranes. But there are several classes of these proteins, and scientists’ knowledge of the structures is almost entirely restricted to just one, the class A subtype. So drugs could be missing a lot of targets. Aim could improve, however, now that researchers have the first two reports of class B GPCR structures (Nature 2013, DOI: 10.1038/nature12357 and10.1038/nature12393). The work provides essential information efforts to design safer, more convenient medications to treat metabolic diseases, mental health, and more.
Class B GPCRs transmit hormone signals from the outside of a cell to the inside. The diabetes drug exenatide and the osteoporosis drug teriparatide, among others, target this protein family. “The issue is that all those drugs are peptides that must be injected,” says Van Andel Research Institute structural biologist Eric H. Xu. Companies have spent years searching for small-molecule, would-be pills to activate these receptors. “They haven’t found one yet,” adds Xu, who wasn’t involved with the new work.
The class B structures explain that struggle: They’re different from class As. The new structures include the antianxiety target corticotropin-releasing factor (CRF) receptor by researchers at Heptares Therapeutics and the diabetes target glucagon receptor by an international team led by Raymond C. Stevens of Scripps Research Institute California.
All GPCR structures have seven α-helices. But compared with prior structures, “class B receptors are much more open, more V-shaped,” says Fiona H. Marshall, Heptares’ chief scientific officer. “That large pocket accommodates water and peptide hormones, which is why it’s hard to get a small molecule to make all the right interactions.”
The newly solved structures reveal additional pockets that may be useful to medicinal chemists, according to an accompanying commentary in Nature by Patrick M. Sexton and Denise Wootten at Australia’s Monash Institute of Pharmaceutical Sciences (2013, DOI: 10.1038/nature12413). In particular, notes Brian Shoichet, who studies molecular recognition in GPCRs at the University of Toronto, the CRF receptor was crystallized with a small-molecule ligand, and that small molecule occupies a deeper binding site than usual, which gives researchers a new location to exploit.
These structures may shed some light on class B more generally, adds Ryan G. Coleman, a postdoctoral researcher on Shoichet’s team. Computer modeling experts might be able to use them to guide discovery of drug candidates, not only for CRF and glucagon receptors, but for related proteins as well, he says.
The structures are not complete, Stevens cautions. It’s been hard to crystallize class B GPCRs because they have a hydrophilic section that resides outside of cells in addition to the hydrophobic membrane-spanning component. Both structures lack the hydrophilic portion. “What you really want is a full-length structure,” he says, “in both inactive and active states.” Both teams say they’re reaching for that goal.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter