Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
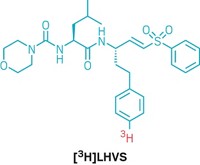
Synthesis
Sidesplitting Nickel Breaks Up Laughing Gas
N-Heterocyclic carbene activates normally inert nitrous oxide to nickel insertion, resulting in rupture of the N–N bond
by Stephen K. Ritter
September 9, 2013
| A version of this story appeared in
Volume 91, Issue 36
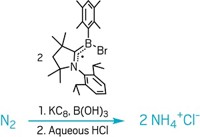
Many people are familiar with laughing gas—nitrous oxide, N2O—for the euphoric effects it produces when inhaled as an anesthetic for dental procedures. The compound’s extreme inertness is what helps make it effective. Chemists have been interested in finding ways to make N2O more reactive to remediate its effects as a greenhouse gas or to use it as a reagent in industrial syntheses in the same way H2, CO, and other small-molecule gases are used. Kay Severin and coworkers at the Swiss Federal Institute of Technology, Lausanne, have devised a process in which a nickel complex inserts itself sidelong into N2O’s N–N bond and cuts it in two (Inorg. Chem. 2013, DOI: 10.1021/ic401524w). The team’s trick to cracking up N2O rests with the reactive power of an N-heterocyclic carbene. When added together, the carbene grabs N2O’s terminal nitrogen, holding the molecule and weakening the N–N bond for a nickel cyclooctadiene complex to attack. The separated N and NO fragments could be released from the resulting dimeric nickel nitrosyl complex or transferred to an organic molecule in a separate step.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter