Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Alcohols’ Tertiary Center Undergoes Stereoinversion
ACS Meeting News: New reaction flips triply substituted alcohols into isonitriles
by Bethany Halford
September 13, 2013
| A version of this story appeared in
Volume 91, Issue 37

It’s turnabout time for triply substituted alcohols in a new reaction, which flips the configuration of an alcohol’s central carbon as the compound is transformed into an isonitrile. The reaction provides a shortcut to compounds with tertiary alkylisonitriles or tertiary alkylamines that could make it easier to synthesize natural products and design new pharmaceuticals and materials.
Students of organic chemistry learn early that the SN2 reaction—in which a nucleophile displaces a leaving group in such a way that the substrate’s stereochemical arrangement is flipped—can’t take place on tertiary carbons. The triply substituted carbon electrophile is simply too crowded for the reaction’s required backside attack.
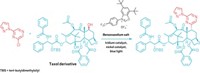
Now chemists have come up with an SN2-like reaction that transforms tertiary alcohols into tertiary alkylisonitriles while inverting the substrate’s stereochemistry (Nature 2013, DOI: 10.1038/nature12472). Ryan A. Shenvi, Sergey V. Pronin, and Christopher A. Reiher of Scripps Research Institute, in La Jolla, Calif., developed the reaction, which makes use of the strong Lewis acid scandium(III) trifluoromethanesulfonate. The substrate alcohols are converted into trifluoroacetate esters, which undergo attack by the nucleophile trimethylsilyl cyanide. The resulting isonitrile can be converted into many other nitrogen-containing functional groups, Shenvi noted.
The work was presented last week at the ACS national meeting in Indianapolis in a symposium held by the Division of Organic Chemistry.
Shenvi told C&EN that he wouldn’t call the transformation an SN2 reaction because about 10% of the product retains its original stereochemical configuration. Rather, Shenvi suspects the reaction forms a transient ion pair that blocks the incoming nucleophile from one side of the substrate.
“The stereocontrolled synthesis of aliphatic amines remains a challenging endeavor, and Shenvi’s work not only provides an excellent solution but does so in a functional-group-tolerant manner with readily accessible reagents and intermediates,” commented John L. Wood, an expert in organic synthesis at Baylor University, in Texas.
Shenvi’s team is currently using the reaction to make antimalarial compounds, but he has bigger plans. The way forward, he said, is to develop an entire arsenal of SN2-like reactions of tertiary alcohols that incorporate sulfur, oxygen, and carbon as well.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter