Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Trapping Droplets In Unusual Shapes
Close crowding of surfactant nanoparticles locks nonequilibrium shapes in place
by Bethany Halford
October 28, 2013
| A version of this story appeared in
Volume 91, Issue 43
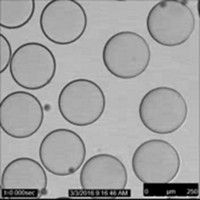
Shake a bottle of oil-and-vinegar-based salad dressing and you’ll soon see spheres at the interface where the two immiscible liquids meet. That’s because a sphere is the most energetically favorable shape for droplets formed by mixing such liquids. The spherical shape minimizes the surface area and thus the interaction between the hydrophobic oil and the hydrophilic vinegar. Scientists at the University of Massachusetts, Amherst, have now come up with a way to distort the shapes of droplets and trap them in unusual forms (Science 2013, DOI: 10.1126/science.1242852). Thomas P. Russell, Mengmeng Cui, and Todd Emrick found that when they combined carboxylated polystyrene nanoparticles with amine-terminated polymers, they could create a nanoparticle surfactant. Typical of surfactants, these nanoparticles gather at the interface of two immiscible liquids, such as a drop of water in silicone oil. When the researchers distorted the shape of the drop, either by applying an electric field or via physical manipulation, they found that more nanoparticles crammed into the interface, locking the new shape in place. “The ability to generate and stabilize liquids with a prescribed shape poses opportunities for reactive liquid systems, packaging, delivery, and storage,” the researchers say.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter