Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Hepatitis C’s Infection Machinery
Structural Biology: First glimpse of a protein used by the deadly virus to infect humans
by Sarah Everts
December 2, 2013
| A version of this story appeared in
Volume 91, Issue 48
\

\
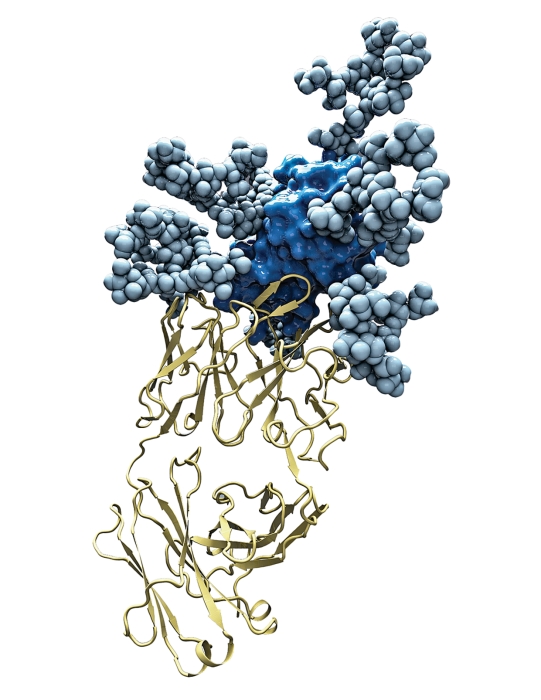
Researchers have solved the crystal structure of E2, a protein used by the hepatitis C virus to infect human cells. A complex (left) of the protein (dark blue), its carbohydrate coat (light blue), and a human antibody (yellow) was crystallized. The protein’s structure by itself is shown above.
The hepatitis C virus kills more people annually in the U.S. than HIV. Over the 24 years since the discoveryof the hepatitis C virus, many researchers have tried and failed to get a crystal structure of the two proteins used by the pathogen to infect its host and cause deadly liver damage. Now a team of scientists led by Ian A. Wilson, Andrew B. Ward, Leopold Kong, and Mansun Law at Scripps Research Institute California are finally reporting a structure of one of these proteins, called the E2 envelope glycoprotein, at 2.65 Å. They used X-ray crystallography and electron microscopy (Science 2013, DOI: 10.1126/science.1243876).
“This is a big step forward in our knowledge of hepatitis C,” comments Michael Houghton, a microbiologist at the University of Alberta who first identified the virus in 1989. “It allows us to understand how the virus binds liver cells. And it will help us in designing vaccines and protective antibodies,” he adds.
The structure may also help drug developers create a new class of hepatitis C therapies that target the E2 protein, Law adds. To date, the drugs on the market or in clinical trials have targeted the pathogen’s polymerase, protease, and phosphoprotein.
The hepatitis C virus, which has infected 2 to 3% of the world’s population, often leaves little trace of its arrival in human hosts—at least initially, Law says. After decades of infection, hepatitis C causes chronic liver disease, followed by liver failure or cancer. Many people discover they are infected only after the liver damage has set in, Law says. This is partly because the virus can be hard to detect in patients because it leaves just small traces of its presence.
Not only is the virus tough to detect in patients, but it has also been extremely hard to study in a lab, comments Alexander Ploss, who works on hepatitis C at Princeton University. After the pathogen’s discovery, it took a decade for scientists to culture it in a lab to get ample quantities for experiments. Animal models for the condition were also a challenge to develop, Ploss explains. Initially chimpanzees were the only animal models, but they were expensive and ultimately phased out for ethical reasons, he says. Only recently have scientists developed rodent-based models for the disease.
Solving the structure of the E2 protein had been incredibly tough because the protein has regions lacking defined structure and is covered in highly branched carbohydrates, Houghton says. So the team “played some very clever tricks,” he says. They modified the length of the protein, removed some of the carbohydrates, and crystallized the resulting protein in the presence of an antibody known to neutralize the pathogen. Then the team went to great lengths to show, using a variety of biochemical techniques, that the truncated, crystallized form is still biologically relevant, Ploss adds.
Although E2 has a β-sandwich and some short α-helices, nearly 62% of the protein has no secondary structure, which explains why it was so tough to crystallize and to solve its structure, Law says. What’s really valuable about the work, Ploss says, is that the team identified regions of the E2 protein that are highly conserved, essential for infection, and not physically blocked by carbohydrates. “This opens up new ways to rationally design new vaccine candidates,” he adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter