Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Discussing Chemical Bonding
December 9, 2013
| A version of this story appeared in
Volume 91, Issue 49
ACS 2012 IRS Form 990 Available
The American Chemical Society’s 2012 Form 990 is now available on ACS’s website. To access the information, go to www.acs.org and follow these instructions: Click on “About Us,” then click on “ACS Financial Information.” Go to the heading “ACS IRS Form 990” and click on “2012 IRS Form 990.”
Please see also the related “Guide to Schedule J” for explanatory information regarding ACS Executive Compensation. If you have any access problems, contact webmaster@acs.org.
Congratulations on “Chemical Connections,” which summarizes the peculiar history of our understanding of the nature of the chemical bond (C&EN, Sept. 9, page 28). I agree with most of the article, except for the passage that connects quantum mechanics with the Gilbert N. Lewis model of chemical bonding. Stephen Ritter writes, “As quantum mechanics and chemical bonding theories would soon show, atoms in molecules are held together by a balancing act of attractive and repulsive forces generated by Lewis’ electron pair, an invisible entity felt but not seen.”
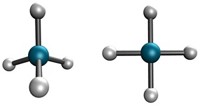
This is not entirely correct. Atoms in molecules are held together by the forces between nuclei and electrons that do not have to come in pairs in order to yield a chemical bond. This becomes obvious by the simplest molecule, H2+, which has only one electron and yet has a strong covalent bond.
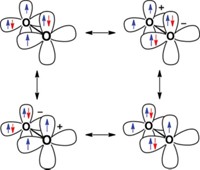
It is a widespread misconception that the nature of covalent bonding lies in the formation of an electron pair. It is true that most stable molecules have even numbers of electrons that can be described in terms of electron pairs as suggested by Lewis, but this is not the basis of chemical bonding. The origin of covalent interactions is the interference (resonance) of the wave functions that describe the interacting electrons. This was pointed out by Walter Heitler and Fritz London in 1927, when they presented the first physically correct description of covalent bonding using the newly developed quantum theories of Werner Heisenberg and Erwin Schrödinger.
The stability of closed-shell molecules and the successful modeling of chemical bonding in terms of electron pairs come from the Pauli postulate, which stipulates that a maximum of two electrons can occupy the same orbital, but this is only a boundary condition for a correct wave function.The Pauli postulate is an important factor for the structure and reactivity of molecules, but it is not the source for chemical bonding.
Chemistry is a sensuous science, and many chemists have difficulties identifying the origin of chemical bonding with an abstract mathematical function. Rather, they prefer the electron-pair model of Lewis, which can become an object of human imagination, albeit with the character of a unicorn that is known to everyone, although no one has ever seen one.
The chemical bond will surely remain a source of controversial discussion for many years.
Gernot Frenking
Marburg
Germany


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter