Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
CO Serves As Its Own Cocatalyst On Gold
The presence of neighboring CO molecules on gold nanoclusters enhances dioxygen oxidation of CO to carbon dioxide
by Mitch Jacoby
February 11, 2013
| A version of this story appeared in
Volume 91, Issue 6
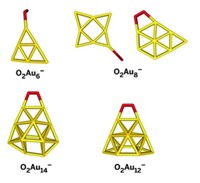
Since the unexpected finding more than 20 years ago that gold, an archetypal inert metal, can serve as a carbon monoxide oxidation catalyst, scientists have searched for the basis of the precious metal’s reactivity. Chemists now know that nanosized gold clusters can catalyze various oxidations, esterifications, and epoxidations, and they have uncovered a few of the mechanistic details. A computational study has found that CO can surprisingly provide a cocatalytic assist to gold nanoclusters during oxidation reactions. This self-oxidation mechanism—uncovered by Xiao Cheng Zeng of the University of Nebraska, Lincoln; Yong Pei of Xiangtan University, in China; and coworkers—reveals a new twist to how gold functions as a catalyst (J. Am. Chem. Soc., DOI: 10.1021/ja309460v). The researchers found that when CO is bound to certain triangular Au3 active sites on gold nanoclusters in the presence of O2, the CO molecule helps facilitate bond scission in an adjacent OCOO intermediate. The analysis shows that an attack on the intermediate by the Au3-bound CO neighbor would significantly accelerate the rate of O–O bond breaking, resulting in formation of two CO2 molecules.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter