Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Current Analytical Methods May Underestimate Levels Of Mercury In The Air
Pollution Monitoring: Amount of reactive mercury in the atmosphere could be two to three times higher than previously reported
by Janet Pelley
January 24, 2013
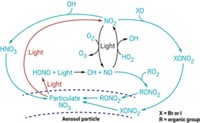
Environmental scientists worry about airborne mercury because when it falls to earth, the metal can transform into a toxic organic form that travels up aquatic food chains, harming wildlife and people. A new study reports that the methods scientists use to monitor mercury in the air underestimate the levels of a reactive form of the metal. This form, which microorganisms readily transform into toxic methylmercury, may be two to three times more abundant in the atmosphere than previously thought, the researchers say (Environ. Sci. Technol., DOI: 10.1021/es3039104).
About 95% of the mercury in the air is elemental mercury, which is relatively inert. The rest consists of reactive mercury(II), which consists of salts such as mercury chloride that bacteria easily convert into methylmercury, says Mae Sexauer Gustin, an environmental scientist at the University of Nevada, Reno.
Scientists keep tabs on both forms so they can model how the metal moves in the environment to assess environmental and human health risks posed by mercury. These measurements help inform policy on regulations such as emissions standards for mining operations and coal-burning plants, two major sources of the airborne metal.
But while elemental mercury is easy to measure, reactive mercury is not. Reactive mercury compounds exist in the air at very low concentrations—in the parts per quadrillion range. These compounds also tend to react with and then stick to the surfaces of equipment used to collect air samples, meaning fewer molecules reach detectors.
The biggest challenge is that scientists haven’t identified all of the types of reactive mercury species in the atmosphere. As a result, the standard method of measuring reactive mercury doesn’t target individual species. Instead it uses potassium chloride-coated quartz to trap mercury compounds that are reactive. Researchers worry that these instruments don’t capture all reactive mercury compounds, because the coated quartz surface may not be ideal to catch every species.
After attending a workshop in 2010 that discussed possible ways around these hurdles, Gustin and her team decided to compare the standard method to a new method developed by colleagues at the University of Washington. In the latter method, instruments collect air samples and measure the total amount of mercury present along with levels of elemental mercury. Then, researchers can determine reactive mercury levels by subtracting the elemental numbers from the total mercury numbers.
The team compared the two methods at a research station outside Reno, Nevada. They connected instruments for both methods to a tube that pulled in air for analysis. In addition to measuring mercury levels in the air around their field site, the researchers also spiked the incoming air with known quantities of reactive mercury.
Gustin’s team found that the U of Washington method reports significantly higher levels of reactive mercury than the standard one does. Based on these differences, they concluded that reactive mercury concentrations in the air could be two to three times greater than previously reported in the literature.
“This study raises the question: Is there something wrong with our measurement techniques?” says Alexandra Steffen, a chemist at Environment Canada, a government agency. She thinks the results support previous worries that the existing method captures only some of the reactive species out there. Parisa A. Ariya, an atmospheric chemist at McGill University, agrees. She thinks that to make accurate measurements, researchers will have to develop methods to characterize all the different reactive species.
Winston Luke, a physical scientist at the National Oceanic and Atmospheric Administration, acknowledges that scientists need to better understand what they’re trying to measure, but cautions that more research is needed before concluding that current techniques underestimate reactive mercury levels. He points out that the tube used to deliver air to the instruments changed the levels of mercury species, in part, through reactions on the tube’s surface. Luke suggests conducting experiments on the instruments side by side without this common tube.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter