Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microbiome
Energy-Storing Nanomaterial Made From Hemp
Electronics: Researchers turn agricultural waste into a carbon nanomaterial for high-power supercapacitors
by Katherine Bourzac
May 15, 2013

Graphene might one day be used in batteries, solar cells, transparent electrodes, and a host of other electronic gadgets. But graphene is still quite expensive to make. Now researchers at the University of Alberta have demonstrated a low-cost process for turning agricultural waste into graphenelike nanomaterials for use in energy storage electronics (ACS Nano 2013, DOI: 10.1021/nn400731g).
With high surface area and conductivity, graphene is ideal for use as electrodes in batteries and supercapacitors, which are energy storage devices that excel at providing quick bursts of power. Supercapacitors charge and discharge faster than batteries can because they store energy in the form of fast-moving charges on the surfaces of their electrodes. Currently, supercapacitors are used in braking systems for buses and fast-charging flashlights.
Commercial supercapacitors use activated carbon electrodes, but experimental devices made with graphene can store more energy. Unfortunately, graphene’s production costs can’t come close to competing with the price for activated carbon, about $40 per kilogram, says University of Alberta chemical engineer David Mitlin.
Part of Mitlin’s research is finding ways to use plant waste as feedstocks for commercial materials. He thought he could transform waste from the cannabis plant (Cannabis sativa) into a carbon nanomaterial that had similar properties to graphene and with a much smaller price tag. The cannabis plant’s notorious use is for producing marijuana, but people also grow the plant to use its fibrous parts for products such as rope, clothing, oil, and plastics. The plants used for these industrial applications are referred to as hemp, and have lower levels of psychoactive compounds. Hemp is relatively inexpensive, since the plant grows rapidly in a variety of climates without the need for fertilizer and pesticides.
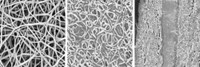
Mitlin and his colleagues focused on a barklike layer of the plant called the bast, which is usually incinerated or sent to landfills during industrial hemp production. “Hemp bast is a nanocomposite made up of layers of lignin, hemicellulose, and crystalline cellulose,” Mitlin says. “If you process it the right way, it separates into nanosheets similar to graphene.”
The Alberta researchers start the process by heating the bast at 180 °C for 24 hours. During this step, the lignin and hemicellulose break down, and the crystalline cellulose begins to carbonize. The researchers then treat the carbonized material with potassium hydroxide and crank up the temperature to 700 to 800 °C, causing it to exfoliate into nanosheets riddled with pores 2 to 5 nm in diameter. These thin, porous materials provide a quick path for charges to move in and out, which is important when a supercapacitor charges and discharges.
The team built a supercapacitor using the nanosheets as electrodes and an ionic liquid as an electrolyte. The best property of the device, Mitlin says, is its maximum power density, a measure of how much power a given mass of the material can produce. At 60 °C, the material puts out 49 kW/kg; activated carbon used in commercial electrodes supplies 17 kW/kg at that temperature.
Liming Dai, a chemical engineer at Case Western Reserve University, says the hempbased material shows promise as a low-cost substitute for graphene. Yury Gogotsi, a materials scientist at Drexel University, sees room for improvement. He points out that the 24-hour high-temperature process will have some associated costs when scaled up. But he’s impressed by this first step. Finding scalable production methods like this one will be key, Gogotsi says, if researchers want to move nanostructured materials out of the lab and into the marketplace.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter