Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Nanoparticle Network Acts As An Artificial Pancreas
Biomedical Engineering: Material releases enough insulin to maintain normal blood sugar levels in diabetic mice for at least 10 days
by Melissae Fellet
May 14, 2013

Patients with type 1 or advanced type 2 diabetes frequently inject themselves with the hormone insulin to maintain their blood sugar levels within a healthy range. Researchers are working on creating implantable or injectable synthetic materials that release insulin in response to glucose levels, mimicking the natural action of the pancreas. A new material made from nanoparticles could improve how quickly these synthetic pancreases respond to glucose levels (ACS Nano 2013, DOI: 10.1021/nn400630x). Materials with fast response times would improve the health and quality of life for people living with diabetes, the researchers say.
Some of these previous insulin delivery methods have used slabs of hydrogels filled with the hormone. To trigger insulin release, researchers added an enzyme called glucose oxidase to the hydrogel. The enzyme consumes glucose and releases an acid. These acidic conditions cause the hydrogel to swell, releasing insulin trapped inside (J. Controlled Release 2008, DOI: 10.1016/j.jconrel.2008.08.009). But these slabs respond slowly to increasing glucose levels, because it takes a long time for the sugar to penetrate fully into the chunks of hydrogel.
Daniel G. Anderson at Massachusetts Institute of Technology and his colleagues wanted an insulin-delivery material that could respond more quickly. Their solution was to trap insulin and glucose oxidase in nanoparticles. They thought that the small size of the particles would increase the amount of enzyme exposed to glucose in the body, making the materials more sensitive to changes in levels of the sugar compared to the hydrogel slabs.
To build these nanoparticles, the researchers reacted the sugar polymer called dextran with ethoxypropene so that the polymer would break down in acid. Then they added the acid-sensitized dextran to a solution containing insulin, glucose oxidase, and another enzyme that helps the glucose oxidase reaction. After stirring the mixture, nanoparticles formed with insulin and enzymes trapped inside.
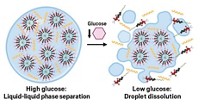
As the particles formed, the researchers added one of two polysaccharide coatings: positively-charged chitosan or negatively-charged alginate. When combined, these two types of nanoparticles formed a three-dimensional network held together by the attractive forces between the oppositely charged coatings. As glucose seeps into the network, the acid released by the glucose oxidase breaks down the polymer particles, releasing insulin.
The team tested the materials by placing them in buffer with different glucose concentrations. After eight hours, the networks placed in buffer with normal human glucose concentrations were still intact and little insulin was released. But in buffer with four times the normal glucose concentrations, the materials completely disintegrated and released most of their hormone cargo in that time.
When the researchers injected the materials into diabetic mice, the particles released enough insulin to maintain normal glucose levels for at least 10 days.
Anderson says that the material needs more work before it’s ready for clinical trials. For example, his team is tuning the material so it responds to changing glucose levels at rates similar to that of the pancreas.
“Developments like this can take the edge off large swings in glucose concentration,” that happen after meals or overnight, says Michael V. Pishko, a biomedical engineer at Texas A&M University. He thinks this material might help people with type 1 diabetes maintain normal blood sugar levels to reduce long-term consequences of the disease, such as blindness, kidney failure, or limb amputation.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter