Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Photonics
Understanding Quantum Dots From The Inside Out
Materials: Tweaking the crystal lattices of the nanocrystals’ cores improves their photonic properties, possibly leading to more efficient light-emitting diodes
by Kate Greene
October 21, 2013

Nanocrystals of semiconductor materials, known as quantum dots, emit a bright glow of a pure color when they’re excited by light or an applied voltage. Some materials scientists have started to exploit these brilliant particles for use in displays as components of light-emitting diodes. Now researchers have found that the nature of the crystal lattices within certain quantum dots dictates how efficiently the particles emit photons when excited (J. Phys. Chem. Lett. 2013, DOI: 10.1021/jz401958u). By tweaking the lattices, the scientists could improve the quantum dots’ efficiencies, which persisted when the dots were integrated into LEDs.
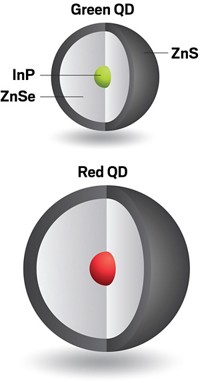
One popular type of quantum dot is a particle with a core made from one material surrounded by a shell of another semiconductor. Quantum dots with thin shells have high quantum yields, the chance that an excited particle emits a photon. Although some types of thin-shell particles have yields near 100%, they tend to lose their efficiency once they’re integrated into a device, making them unattractive for commercialization. The nanocrystals with thick shells have historically had relatively low quantum yields, but they don’t experience a drop in efficiency in devices. So researchers looking to make more efficient quantum dot devices, such as LEDs, have tried to find ways to increase the thick-shell particles’ quantum yields.
Ranjani Viswanatha, a chemist at the Jawaharlal Nehru Centre for Advanced Scientific Research, in India, and her colleagues wanted to explore how the crystal structure of quantum dots affected their quantum yields. They studied dots containing a core of cadmium selenide (CdSe) and a surrounding shell of cadmium sulfide (CdS). Since the crystal structures of CdSe and CdS don’t perfectly line up, there is usually a sharp boundary between the core and the shell of these particles. Previous calculations based on computational models of the particles suggested that a less abrupt transition between the two crystal lattices would lead to higher quantum yields. Viswanatha and her team decided to modify the surfaces of their cadmium selenide cores to look for surfaces that produced high quantum yields.
The researchers made these modifications by allowing for defects to form on the cores’ surfaces, essentially making them rougher. They controlled the process by using various concentrations of surface ligands when synthesizing the CdSe cores.
Unexpectedly, the cores with the most defects created the most desirable boundary with the shell, ultimately leading to the most efficient quantum dots. These defects allowed the crystal lattices to blend together better, enabling a smoother boundary within the quantum dot. “The counterintuitive part of doing this is that you start with a horrible core and end up with a beautiful shell structure,” says Viswanatha.
With the core defects, the nanocrystals’ quantum yields were as high as 94%. Dots with fewer core defects and therefore a more sharp transition between the core and the shell peaked at only 40%.
Traditionally, when researchers make quantum dot LEDs, they add a layer of a material that injects electrons into the dots. This electron injection compensates for low quantum yields and improves the devices’ performance. But when Viswanatha and her group made LEDs with their quantum dots, they didn’t need this extra material. Their devices emitted just as much light with the same amount of power as LEDs made with the electron-injecting material and quantum dots with abrupt core-shell boundaries.
Seth Coe-Sullivan, founder and chief technology officer of QD Vision, a company in Lexington, Mass., that makes quantum-dot-based products, says the group has provided valuable data on how the internal structure of the quantum dots affects their light-emitting properties. He says the field, surprisingly, “only lightly explored” the topic before this.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter