Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Rhodium-Catalyzed Route Builds Three-Membered Nitrogen Rings From Olefins
Chemists synthesize common building block found in nature and in medicine in one step
by Carmen Drahl
January 6, 2014
| A version of this story appeared in
Volume 92, Issue 1
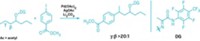
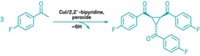
When it comes to synthetic building blocks, the triangular nitrogen heterocycle known as aziridine has plenty going for it. Because of its geometry, aziridine’s strained ring makes it a ready participant in reactions that build complex molecules. For example, the ring appears in bioactive natural products, including chemotherapy drug mitomycin C. Yet with few exceptions, aziridination methods require a protecting group on the nitrogen, which can be tough to remove while keeping the aziridine ring intact. A multilab team has now reported a one-step method that transforms olefins into N–H and N–CH3 aziridines under mild conditions (Science 2013, DOI: 10.1126/science.1245727). László Kürti and John R. Falck of the University of Texas Southwestern Medical Center and Daniel H. Ess of Brigham Young University had previously explored reactions using the aminating agent O-(2,4-dinitrophenyl)hydroxylamine, which they now use for aziridination. They also found that a rhodium catalyst and 2,2,2-trifluoroethanol solvent are essential. Their approach works on hindered olefins and doesn’t disturb sensitive functional groups. The method isn’t enantioselective yet, Kürti says, but the team is using the Ess lab’s density functional theory calculations to hunt for the right catalyst for that job.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter