Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Reaction Assembles Peptide Conjugates With Natural Amide Linkages
Patent-pending process could be used to customize activity of peptide drugs
by Carmen Drahl
April 21, 2014
| A version of this story appeared in
Volume 92, Issue 16
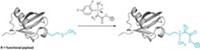
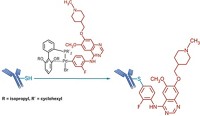
Linking peptide therapeutics to molecules such as polyethylene glycols (PEGs) is a useful way to control their bioactivity, so drugmakers constantly seek new ways to build so-called peptide conjugates. The well-known click reaction, a copper-catalyzed cycloaddition of azides and alkynes, rapidly attaches moieties to peptides without disturbing unprotected functional groups. However, it generates an unnatural linkage and can require an excess of one reagent, an impractical option when some PEGs cost as much as their peptide partners. To address these challenges, Jeffrey W. Bode and colleagues at ETH Zurich have developed a reaction that forms amides from the union of potassium acyltrifluoroborates to O-carbamoylhydroxylamines incorporated into peptides (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja5018442). The reaction works in water with a 1:1 ratio of reagents. Bode’s team used it to attach biotin, PEG, a fluorescent dye, or a lipid to an antidiabetes peptide. “This bioorthogonal reaction is distinguished because it is both fast and also produces native amide bonds,” says the University of Delaware’s Joseph M. Fox, who has also developed protein-tagging methods. ETH has filed a patent application covering PEG attachment with this chemistry.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter