Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Double-Crossed Pyridines
Chemists develop and combine new transition-metal-free cross-coupling reactions to prepare polyfunctional pyridines
by Stephen K. Ritter
May 12, 2014
| A version of this story appeared in
Volume 92, Issue 19
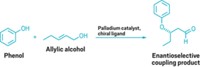
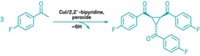
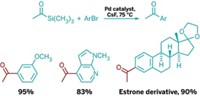
Palladium- and nickel-catalyzed cross-coupling reactions are at the top of the list for many chemists when it comes to functionalizing aromatic rings. But there are other ways. Exploring the options, a team led by Paul Knochel of Ludwig Maximilian University of Munich, in Germany, has developed a pair of transition-metal-free cross-couplings facilitated by the Lewis acid adduct BF3•O(CH2CH3)2 (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201400750). In one case, Knochel, Quan Chen, and Thierry León carried out direct oxidative alkynylations of 4-substituted pyridines using alkynyllithium reagents to form 2,4-disubstituted pyridines. In the second case, they performed nonoxidative alkylations of 4-substituted pyridines using an alkylmagnesium reagent. The team realized they could combine the two approaches in tandem syntheses to prepare even more elaborately substituted pyridines (one example shown). Knochel’s group is now using the cross-coupling reactions to functionalize other nitrogen heterocycles and plans to apply the chemistry to natural product synthesis.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter