Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Protein Target Of Clot-Preventing Drugs Seen For The First Time
P2Y12 receptor’s X-ray structure reveals protein pockets that may prove useful in drug design
by Carmen Drahl
May 12, 2014
| A version of this story appeared in
Volume 92, Issue 19
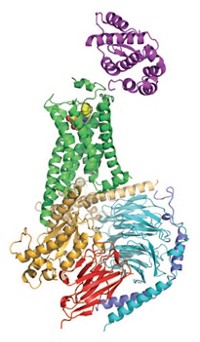
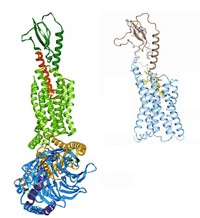
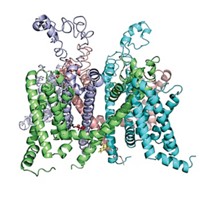
The blockbuster blood-clot-preventing medication Plavix (clopidogrel) was discovered before researchers knew much about its biochemical target, which is a protein receptor called P2Y12. Today, however, they can at last see P2Y12’s structure, which could lead to next-generation drugs. Qiang Zhao of Shanghai Institute of Materia Medica teamed with Raymond C. Stevens of Scripps Research Institute California, Kenneth A. Jacobson of NIH, and their colleagues to solve P2Y12’s crystal structure (Nature 2014, DOI: 10.1038/nature13083 and 10.1038/nature13288). The researchers knew that P2Y12 is a G protein-coupled receptor, a protein that snakes across the cell membrane seven times. But they didn’t know that the conformation of its extracellular protein loops differs dramatically depending on whether P2Y12 is binding to an activator or an inactivator. What’s more, P2Y12 has two subpockets in its binding site, and none of the drugs that target the receptor takes advantage of both. Jacobson notes that chemists are seeking next-generation clot fighters that carry a lower risk of bleeding than established drugs. “Without these structures, we were working in the dark,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter