Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Bismuth Catalyzes CO2 Reduction
by Craig Bettenhausen
May 19, 2014
| A version of this story appeared in
Volume 92, Issue 20
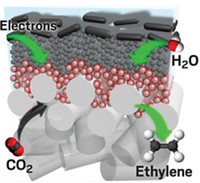
Inexpensive bismuth may soon give chemists a new option for dealing with carbon dioxide captured from industrial processes. Joel Rosenthal and coworkers at the University of Delaware have developed a non-precious-metal electrocatalyst that “efficiently and selectively reduces CO2 to CO, which is an important energy-rich commodity chemical that can be used to synthesize liquid fuels,” Rosenthal says. Using bismuth(III) triflate as a precursor, the researchers electrodeposited amorphous bismuth metal on a carbon electrode under mild conditions. The bismuth catalyst achieves current densities that are an order of magnitude greater than existing silver and gold CO2-reduction catalysts (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja501923g). The high current density means that a device using the catalyst can churn out a large volume of CO per electrode area. Clifford P. Kubiak, an expert in catalytic CO2 fixation at the University of California, San Diego, says that although challenges remain with the system, such as the high operating potential and the coproduction of water with the CO, “this is a very important contribution to the field of electrochemical reduction of CO2.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter