Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Rethinking Photocatalysis
Catalysis: Study questions conventionally ascribed role of metal particles on semiconductors
by Mitch Jacoby
May 23, 2014
| A version of this story appeared in
Volume 92, Issue 21

Titanium dioxide’s knack for mediating sunlight-driven chemical reactions has made it the material of choice for a host of applications, including self-cleaning glass, antifogging coatings, and splitting water to make hydrogen. Researchers have known for years that modifying the semiconductor with metal nanoparticles increases its photocatalytic activity. But the conventional explanation for that enhancement has been called into question by a new study that challenges the mechanistic role of the metal nanoparticles.
The study may deepen understanding of photochemical processes and may lead to lower-cost catalysts.
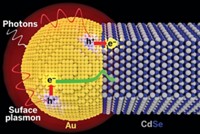
Shining light on TiO2 or other semiconductors promotes electrons to an excited state from which they can stimulate chemical reactions. Much of the time, the electrons shed their excess energy far too quickly for reactions to commence. But if TiO2 is doped with platinum or other metal nanoparticles, excited electrons can quickly hop onto the metals and get trapped before they can cool down, according to the common explanation.
In the case of water splitting, that model indicates that the metals serve as tiny islands on the semiconductor surface on which hydrogen ions can congregate, undergo reduction with trapped electrons, and form H2.
A team led by University of California, Riverside, chemistry professor Francisco Zaera now proposes that electron transfer from the semiconductor to the metal may not play a significant role in photocatalysis. The team came to that conclusion by using transient-absorption and time-resolved-fluorescence spectroscopy methods to probe the photophysics and energy-transfer processes in a series of custom-made nanoparticles, including Au/TiO2 and Pt/TiO2 (Proc. Natl. Acad. Sci. USA 2014, DOI: 10.1073/pnas.1405365111).
The Riverside team proposes that in contrast to the electron-trap model, excited electrons stimulate H+ reduction on the surface of the semiconductor, not the metal. Hydrogen atoms then migrate from the semiconductor to the metal, which combines them catalytically to form H2.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter