Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Probes Search Live Cyanobacteria For Biofuel-Production-Boosting Proteins
ACS Meeting News: Strategy may uncover new target proteins for bioengineering
by Carmen Drahl
August 14, 2014
| A version of this story appeared in
Volume 92, Issue 33


For something so tiny and brainless, cyanobacteria have proven awfully hard to push around. The blue-green microbes are promising hosts for biofuel production. Yet attempts to boost output by engineering the microbes’ genomes rarely work in big bioreactors. Researchers at Pacific Northwest National Laboratory think setbacks happen because the biochemistry that regulates fuel compound production isn’t fully understood. At the American Chemical Society national meeting in San Francisco, they presented molecular probes that might help close that knowledge gap.
The probes reveal metabolic pinch points regulated by oxidation and reduction of cysteine side chains on enzymes. Such reactions are commonplace and help cells respond to their environment, including the stresses of being coaxed to make biofuels. The probes covalently label reactive cysteine amino acids on proteins inside living cyanobacteria, but only when the cysteine thiol group is in its reduced form. “We want to identify proteins where redox chemistry acts like a switch, and where keeping that switch on keeps production of your hydrogen or alkane fuel flowing,” said Aaron T. Wright, who led the research.

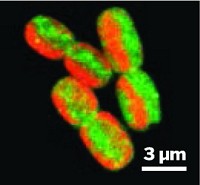
Natalie C. Sadler, who presented the work in the Division of Biological Chemistry, and her colleagues used confocal microscopy to demonstrate that the probes enter Synechococcus cyanobacteria. They starved the microbes of nutrients or, because the microbes use photosynthesis, switched between light and dark. Both are extreme instances of what might happen inside a bioreactor. The team tracked changes over time in reduced and oxidized cysteines. Their list of redox-sensitive proteins includes several enzymes already known to be redox-regulated, but others on the list, including gene transcription regulators, were not (Front. Microbiol. 2014, DOI: 10.3389/fmicb.2014.00325; ACS Chem. Biol. 2014, DOI: 10.1021/cb400769v).
Kate Carroll, a protein redox regulation expert at Scripps Florida, said the research unearths “an exciting array of protein targets” for making better cyanobacteria-based biofuel factories.
Not all the enzymes the team found are necessarily major metabolic switches, Wright said. “So now we’re dying to dig in and see which ones truly are.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter