Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
A Step Toward Making Painkillers Without Poppies
Bioengineering: Modified yeast produce morphine and semisynthetic opioids starting from thebaine
by Celia Henry Arnaud
August 28, 2014
| A version of this story appeared in
Volume 92, Issue 35

The supply chain for some of the world’s most prescribed painkillers—natural opioids such as morphine and semisynthetic opioids such as oxycodone—depends on the cultivation of opium poppies, Papaver somniferum. Although poppy farming is a relatively cheap way to obtain the needed materials, it risks diversion to illicit drugs.
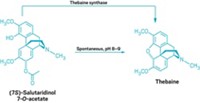
Researchers at Stanford University are working toward the goal of poppy-free production of these painkillers. Christina D. Smolke and coworkers have engineered yeast that can biosynthesize natural and semisynthetic opioids from thebaine, a starting material obtained from poppies (Nat. Chem. Biol. 2014, DOI: 10.1038/nchembio.1613). Previously, they engineered yeast to produce salutaridine, an earlier intermediate on the path to morphine. They are working to develop the remaining two steps needed to connect the two parts of the overall pathway.
Smolke, postdoc Kate Thodey, and graduate student Stephanie Galanie engineered into yeast the P. somniferum genes that encode the enzymes the flowers use to synthesize morphine. To make the semisynthetic opioids, they also engineered into the yeast genes from bacteria that metabolize natural opiates.
But they were surprised when they looked at what the yeast made—an undesired by-product called neomorphine. The unexpected product was a result of the promiscuity of the enzymes, Smolke explains.
To steer the yeast away from the neomorphine pathway and toward the desired morphine and codeine products, Smolke and coworkers separated the enzymes in the cell. They tagged the enzymes with peptides that would traffic them to specific compartments within the cell, such as the endoplasmic reticulum. In this way, they were able to make the enzymes see the right intermediates in the right order. “This strategy redirected the flux to natural opiates we wanted to produce,” Smolke says.
By adjusting the number of copies of enzymes and the amount of different substrates they fed the yeast, Smolke and coworkers were able to get the total opioid concentration up to 131 mg/L.
That’s far less than would be needed for commercial production, Smolke concedes. “One of the big challenges will be making sure that the efficiencies and activities of the enzymes at each step are high enough to get sufficient flux through the pathway to make this commercially viable,” she says. Even after the researchers integrate the pathways, they will have to optimize the pathway, the yeast strain, and the fermentation conditions.
“It’s an extreme challenge to go from a primary metabolite in a microbe all the way through to a molecule like morphine,” says Toni Kutchan, vice president for research at the Donald Danforth Plant Science Center in St. Louis. Smolke’s “not there yet, but she’s doing a very good job.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter