Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Diamond-like Nanothreads Form Under Extreme Pressure
Molecular Discovery: Slow compression and decompression of benzene yields new class of crystalline nanomaterial
by Lauren K. Wolf
September 26, 2014
| A version of this story appeared in
Volume 92, Issue 39

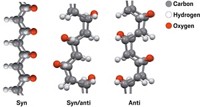
Using a high-pressure device at Oak Ridge National Laboratory, graduate student Thomas C. Fitzgibbons recently transformed a few cubic millimeters of liquid benzene into a white, crystalline powder. The solid was made of a substance never before observed: ultrathin threads of carbon atoms bound to one another in a pattern resembling diamond (Nat. Mater. 2014, DOI: 10.1038/nmat4088).
The threads, Fitzgibbons and colleagues hope, might one day be used to make strong, lightweight materials for vehicles and other objects.
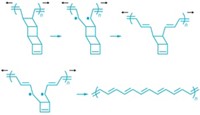
Creating ordered nanomaterials from organic molecules such as benzene has been a longtime goal of the group, says John V. Badding, who is Fitzgibbons’s Ph.D. adviser at Pennsylvania State University. In the past, when Badding and his team put the squeeze on benzene, they produced only disordered substances, though. This time, crystalline order seems to have arisen because “the apparatus Tom used required us to compress and decompress slowly,” Badding explains.
Fitzgibbons ramped up to a pressure 200,000 times that of Earth’s atmosphere and back down over 20 hours. This glacial pace, the team proposes, allowed stacks of benzenes to polymerize slowly enough to form the 6-Å-thick ordered threads (structure shown).
“This is pioneering work,” says Roald Hoffmann, a theoretical chemist at Cornell University. Hoffmann, who collaborates with Badding, predicted a structure related to that of the nanothreads while studying benzene polymerization a few years ago.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter