Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Patent Picks: Perovskite Solar Cells
A look at recent patenting activity in perovskite solar cells, brought to you by C&EN and CAS
by Amanda Yarnell
December 15, 2014
| A version of this story appeared in
Volume 92, Issue 50
Jump to Topics:
- Perovskite Layer Lets Solar Cells Flex
- Metal Mesh Promises To Lower Costs
- Yttrium Doping Drives Up Efficiencies

Silicon-based solar cells, the gold standard in solar panels, are expensive to make, requiring costly starting materials and clean rooms for manufacturing. In the 1980s, scientists explored whether dye-sensitized solar cells could compete with silicon-based ones. Dye-sensitized cells were cheaper to manufacture, but their efficiency in converting light into electricity couldn’t be pushed beyond 10%—a far cry from silicon’s 25%. Then, in 2009, a Japanese team reported solid-state versions of liquid electrolyte dye-sensitized solar cells that incorporated organometallic trihalides featuring the perovskite crystal structure. The efficiencies of such perovskite-based solar cells were initially under 4%. But today’s best perovskite-based solar cells boast efficiencies on the order of 20%. Their commercial promise is evident in the sudden burst of activity in patent applications seen in the Chemical Abstracts Service databases in recent years. Three such patent applications are featured here.

Seeking to manufacture commercial materials such as solar window coatings and solar panels that will let buildings generate their own electricity, Henry J. Snaith and coworkers at the University of Oxford are working to develop solar cells that are efficient, flexible, and transparent. One of their most promising solar-cell designs incorporates thin films of light-absorbing perovskites between n-type and p-type semiconductor layers (WO 2014045021). Such cells convert light to electricity with efficiencies of 7.5%, without the need for the porous metal oxide composite layer that’s normally required in perovskite-based solar cells. Eliminating that layer yields cells that are lighter weight and more flexible than standard perovskite-based ones, Snaith says. To make their CH3NH3PbI2Cl perovskite films, Snaith’s team combines CH3NH3I and PbCl2 via vapor deposition. The method allows them to control the evaporation rate of each component such that they can produce CH3NH3PbI2Cl perovskite films of the desired thickness. Oxford Photovoltaics is now commercializing the work. Snaith, who founded the company and is its chief scientific officer, says that it aims to make the flexible cells commercially available in 2017.
Conventional perovskite solar cells are typically fabricated by sandwiching a perovskite-coated semiconductive titanium oxide layer and an organic electrolyte solution between two transparent conducting glass electrodes. Light passing through the transparent electrode onto the layer of photosensitive perovskite material stimulates electron movement, resulting in an electrical current. A new cell design from Michael J. Tauber of the University of California, San Diego, and colleagues does away with the costly transparent conductive oxides found in solar cells’ electrodes (WO 2014165830). The device comprises a cathode with a metal mesh that is optically transmissive and electrically conductive and an anode including a metal base layer that is optically opaque and electrically conductive. Transparent conductive oxides are one of the most costly components of a solar cell, so avoiding the material could significantly reduce the overall cost of perovskite-based photovoltaics, the authors suggest.
↑ Top
Jump to Topics:
- Perovskite Layer Lets Solar Cells Flex
- Metal Mesh Promises To Lower Costs
- Yttrium Doping Drives Up Efficiencies
Patent Picks is a collaborative effort by C&EN and CAS. This feature reports on trends CAS scientists observe from patents in CAS databases. Patents now generate more than 70% of the new substances appearing in the literature.
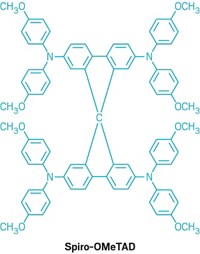
To increase the efficiencies with which perovskite-based solar cells convert light to electricity, Michael Grätzel of the Swiss Federal Institute of Technology (ETH), Lausanne, is focusing on doping such cells’ semiconductor layers. Grätzel’s team recently reported that doping the TiO2 semiconductor layer with 0.5% yttrium boosts efficiencies to 11.2%, compared with 10.5% for the nondoped equivalent (WO 2014180780). The researchers also report that their solar cells can be manufactured without the need for a liquid electrolyte, which eliminates solvent evaporation and water leakage that decrease the stability of conventional solar cells. Instead they use a solid organic hole-conducting electrolyte made from the polyaromatic ring compound spiro-OMeTAD. The technology is being commercialized by Australian photovoltaics firm Dyesol.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter