Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
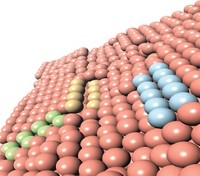
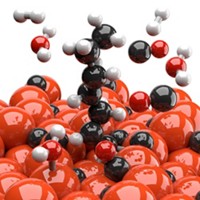
Bimetal Catalyst Makes Methanol From Low-Pressure Carbon Dioxide
Computational studies plus experiments turn up catalytically active nickel-gallium alloys for converting carbon dioxide to methanol
by Mitch Jacoby
March 3, 2014
| A version of this story appeared in
Volume 92, Issue 9
If methanol could be made where and when it’s needed by reacting CO2 with hydrogen obtained from sun-driven water splitting, the simple alcohol could serve as a low-cost sustainable fuel and chemical feedstock. That possibility may be closer to reality by the discovery of a nickel-gallium methanol synthesis catalyst that works well under mild conditions (Nat. Chem. 2014, DOI: 10.1038/nchem.1873). Methanol is currently made in large industrial facilities from a mixture of CO, CO2, and petroleum-derived hydrogen. The commercial process is typically driven by a Cu/ZnO catalyst at high pressure. Altering the conditions reduces the methanol yield and can lead to a high concentration of unwanted CO. By using a computational technique, a team led by Jens K. Nørskov of Stanford University and Ib Chorkendorff of the Technical University of Denmark identified a family of Ni-Ga alloys with promising catalytic properties. They synthesized and tested several of the materials and report that Ni5Ga3 converts CO2 and hydrogen to methanol at atmospheric pressure as well as or better than Cu/ZnO and produces lower levels of CO.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter