Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Natural Products
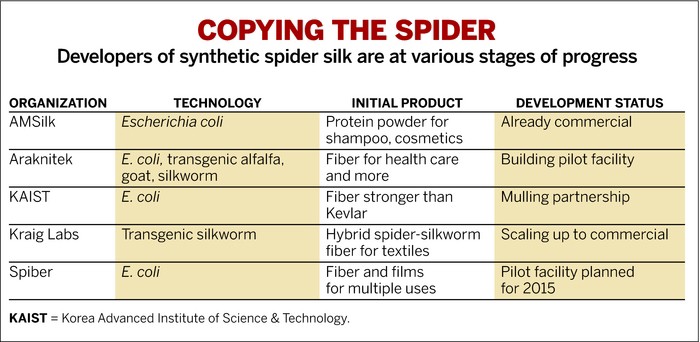
Spider Silk Poised For Commercial Entry
Biotech firms overcome technical challenges to make fiber stronger than steel and tougher than Kevlar
by Alex Scott
March 3, 2014
| A version of this story appeared in
Volume 92, Issue 9

There is a scene in the film “Spider-Man 2” where Spider-Man prevents a train full of people from crashing by holding it back with about 10 sets of spider silk ropes each less than half an inch thick. It turns out the scene isn’t just fantasy.
“We calculated roughly how thick the fibers were, how many of them he had attached to the walls, how much the locomotive and people weighed, and how fast it appeared to be going,” says Randy Lewis, a professor of biology and biological engineering at Utah State University. “Spider-Man would have been able to stop that train,” says Lewis, a molecular biologist, materials scientist, and chemist who for 25 years has been striving to synthesize spider silk.
Despite being a protein, spider silk is by weight five times stronger than steel and three times tougher than Kevlar, a p-aramid fiber from DuPont. Strength is defined as the weight a material can bear, and toughness is the amount of kinetic energy it can absorb without breaking. The silk’s primary structure is its amino acid sequence, mainly consisting of repeated glycine and alanine blocks.
Potential applications include cables and bulletproof vests. Spider silk’s antimicrobial properties make it suitable for wound patches. Because the silk is not rejected by the human body, it can be used to manufacture artificial tendons or to coat implants. And its thermal conductivity is similar to that of copper but its mass density is one-seventh of copper’s, making it a potential heat management material.
However, gathering the silk from farm-raised spiders, which are territorial and cannibalistic, is not an option. Firms attempting to make spider silk synthetically have copied relevant genes from spiders and inserted them into organisms, such as Escherichia coli, that can express the protein. The protein, though, is complex, and producing silk that is as strong as nature’s has proven elusive.
Further challenges faced by would-be producers are that the protein is insoluble in water and the fiber is so fine—1,500 strands are needed to make a thread—that firms have had to invent new spinning systems. After years of trying to develop commercial spider silk, big companies including DuPont and BASF have dropped out, with the latter pulling the plug on its research just last year.
A handful of much smaller companies are now claiming progress. In the past few months, the German firm AMSilk has begun selling spider silk protein to producers of shampoos and cosmetics. And several other small firms are trying to commercialize their own versions of spider silk. Although the efforts are nascent, it’s starting to look like spider silk protein can be made at a cost that makes it attractive for certain applications. Still unclear is whether spider silk can be produced cheaply and in high enough volume to make Spider-Man’s superstrong cables a reality.
Initial uses for AMSilk’s protein are in nonfiber applications including cosmetics, where spider silk makes the skin feel “supersmooth,” according to the firm, and in shampoos, where it adheres to keratin, the primary structural protein in hair, to make damaged hair feel and look more silky. In May, the company intends to introduce wound-healing spray.
AMSilk is using genetically engineered E. coli to express the protein in a fermentation process. The firm is incorporating four silk varieties into its initial products. The four are derived from the DNA sequence of the European garden cross spider, but AMSilk has engineered specific versions of E.coli to generate about 20 different silk grades at its labs near Munich.
The company is already outsourcing production and says it can increase output as required. “This is scalable technology,” Managing Director Axel H. Leimer says. “If someone ordered 1 ton, we could make it. We have already made a half a ton,” he says. For the first time, a company is producing recombinant spider silk that is as tough and strong as the silk from a spider, Leimer claims.
Leimer is hoping that AMSilk’s know-how will enable it to roll out products generating annual sales in the next couple of years of more than $10 million. He is targeting sales of more than $100 million once large-scale production of synthetic fibers is under way.
Venture-capital-backed AMSilk is a start-up with 25 staffers, of whom 22 are scientists. So far the firm has invested about $16 million, including grants, to develop its process. The technology was developed by Thomas Scheibel, professor of biomaterials at Bayreuth University, in Germany, and a member of AMSilk’s advisory board.
Leimer hopes that, by the end of 2015, AMSilk’s spider silk protein will also be on the market as a dip coating for silicone breast implants. The protein changes the properties of the implant, making its hydrophobic surface slightly negatively charged and reducing the body’s reaction to it, Leimer says. “It’s a double-digit million-dollar market for spider silk.”
Large-scale output for products such as fibers and high-performance textiles will come after 2015, Leimer says. Longer term, AMSilk is eyeing capsules for drug delivery and miniature spider silk beads for vaccines that would get around the need for refrigeration.
Leimer is convinced that, even from the outset, the market will be able to support several players. That is good news for the pack of companies developing spider silk, including Lansing, Mich.-based Kraig Biocraft Laboratories, which is scaling up a process to manufacture a hybrid spider-silkworm silk fiber from transgenic silkworms. Last October, Kraig Labs reached an agreement to codevelop and commercialize the silk with textiles firm Warwick Mills.
Kim K. Thompson, Kraig Labs’ founder and chief executive officer, set out to make spider silk about 10 years ago as a businessman with an idea and a passion for science but no university science education. He approached academics at nearby Michigan State University with the idea to produce spider silk from transgenic silkworms but was told his plan had several major scientific flaws.
Thompson then read a paper by Malcolm J. Fraser Jr., a biology professor at the University of Notre Dame, detailing a process for transposing genes from one organism to another. A few days later, Thompson was in Fraser’s office, where the two men hammered out a viable approach for making spider silk.
Thompson founded Kraig Labs with only $500,000. He is the firm’s only full-time employee; scientific research is primarily outsourced to Fraser and a handful of Fraser’s researchers. “I squeezed more science out of every dollar invested in this project than probably any science development project ever done,” says Thompson, who despite the financial challenges of the early years can’t help but enthuse about the scientific process he has been involved in.
Thompson’s selection of silkworms as hosts for the spider gene has enabled Kraig Labs to jump straight to fiber production in a single step. He is positioning the business to provide a hybrid spider-and-worm silk that is superior to silkworm silk for high-quality textile applications but costs the same. The global raw-silk market is worth $5 billion annually, but the price of the spider silk has to be right, “and this is the cheapest way of making it,” Thompson maintains.
Pilot studies have been successful, and the firm is now scaling up production. Thompson won’t disclose production capacity or location, but he says he plans to farm millions of silkworms for their cocoon silk. Thompson is already discussing a second facility in Vietnam, which has a thriving silkworm industry.
Although silkworms have been raised in Asia for millennia, South Korea’s Korea Advanced Institute of Science & Technology (KAIST) and Spiber, a Japanese start-up, are both developing E. coli spider silk processes. The two firms have yet to place their products on the market, but both are confident that their processes are close to becoming commercially viable.
Spiber has raised $8 million from Japanese investment banks to develop a process for generating spider silk from engineered bacteria. The company plans to open a pilot facility by 2015 capable of producing 100 kg of spider silk fiber per month. Spiber says a single gram of its protein produces about 5.6 miles of artificial silk.
Sang Yup Lee, who leads synthetic spider silk research at KAIST, says he is developing an E. coli-based process for a high-molecular-weight silk that is stronger than Kevlar. Packing enough glycine into the protein has been a major challenge that Lee and his colleagues have had to overcome. Lee tells C&EN he is now at the stage where he is considering forming a partnership with a company to manufacture spider silk commercially.
Utah State’s Lewis is hedging his bets. He and his colleagues are developing four different hosts that carry the spider silk genes: goats, silkworms, E. coli, and alfalfa. In association with Lewis, Utah State founded spin-off company Araknitek in 2012 to commercialize the technologies.
Lewis’s herd of transgenic goats on the university farm carry a gene from orb weaver spiders for making dragline silk, the strongest type of spider silk and the one that the spider uses to make an escape line. The goats make the silk protein in their milk; Lewis isolates it via simple purification. The spider-goat approach is perfect for small-volume medical applications such as sutures and artificial ligaments, he says. “It has the advantage from a regulatory perspective that transgenic animals have already been used to make health care products.”
Lewis will begin commercial tests on the suture thread in the next few months. Advantages over existing silkworm thread are that the spider silk is about 50% finer and doesn’t have the straggly fiber ends disliked by medical professionals, according to Lewis.
His team also has inserted the spider silk gene into a silkworm. This “is the ideal way to go,” Lewis says. But he is not yet convinced that his silkworms will make a high enough ratio of spider silk to silkworm silk. Currently, “we have superior silkworm silk but not spider silk.”
Scaling up production from transgenic animals could, however, prove problematic. “If I was investing in a spider silk company, I would look at classical biotechnology such as E. coli systems,” says Andrea Ricci, a senior analyst at RobecoSAM, a Swiss asset management company that invests in innovative material technologies. “Scaling up is pretty easy. But scaling up with goats or worms poses a completely different outlook.” Ricci is confident that synthetic spider silk could replace materials such as steel in a range of applications but warns that commercial success will depend on getting the cost right.
Of Lewis’s projects, the one using alfalfa engineered to carry the spider silk gene is furthest from being commercially viable. It could be used to make spider silk fibers for volume applications such as textiles for sports equipment. But processing and purifying the protein from the alfalfa remains a major hurdle, Lewis acknowledges.
For Araknitek, E. coli is the near-term option. In January Lewis’s team increased the expression of spider silk in E. coli by a factor of five. And he anticipates being able to increase it by a factor of five again in the near term. The project is now producing enough silk protein for application testing, and Utah State is building a pilot facility with two 500-L fermentation tanks. The E. coli approach would be suited for making large-volume products such as spider silk rope, Lewis says.
Lewis and his team are working on making fibers, films, and gels. Lewis says he is talking with producers of sutures, tires, textiles, automobile air bags, and medical implants. He has even been asked whether his material could replace Kevlar in sails for the America’s Cup race.
Although Lewis’s math showed that Spider-Man’s silk would have been strong enough to stop the commuter train, it also demonstrated that Spider-Man couldn’t have eaten nearly enough to generate all that webbing. That’s fine for the movies. But Araknitek, AMSilk, and the other firms involved are determined that spider silk supplies will soon be ample in the real world.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter