Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Synthetic Polysaccharide Stabilizes Proteins
Biochemistry: Polymer consisting of amide-linked glucose monomers outperforms a natural disaccharide in protecting enzyme during freeze-drying
by Puneet Kollipara
July 9, 2014

Chemists have synthesized a novel class of carbohydrate polymers that could help retain the function of enzymes and protein drugs during storage (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja5036804).
When researchers store proteins, they often freeze-dry them. To protect the biomolecules from the freezing and thawing process, biochemists add saccharides such as trehalose to stabilize the protein structures. These stabilizers envelope the protein and promote hydrogen bonding between it and water molecules. In the case of polysaccharides, the length of the sugar chain can influence how well they stabilize a protein by altering how much of the polymer wraps around it, as well as where and how much hydrogen bonding occurs. But with natural saccharide stabilizers, researchers have no control over the chain length or the number of hydrogen-bonding interactions.
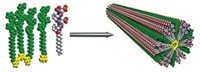
So Mark W. Grinstaff and his colleagues at Boston University developed new polymers that give them that control. Their new polymers, called poly-amido-saccharides, consist of amide-linked glucose monomers. After polymerizing chains of 20 to 50 monomers, the chemists oxidize alcohols on the sugar molecules to produce negatively charged polymers.
The researchers compared their polymers to other stabilizers, including trehalose, which is a disaccharide. They paired each stabilizer with a batch of the enzyme lysozyme and then tested the protein’s activity after 10 freeze-thaw cycles. With trehalose, the enzyme lost about 40% of its pre-storage activity. But with the new polymers, the protein lost less than 20%. The synthetic polymers performed as well as two other natural stabilizers, sodium alginate and sodium hyaluronate.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter