Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Leaves Inspire Photon Energy Upconversion Material
Materials Science: A cellulose-based material protects light-sensitive dye molecules from oxygen damage and could help boost the efficiency of some solar cells
by Jessica Morrison
August 7, 2014

A new material that mimics the structure of a leaf helps light-sensitive dyes convert low-energy light into high-energy photons, a process known as upconversion (ACS Nano 2014, DOI: 10.1021/nn502496a). The leaflike nanopaper protects such dyes from oxygen damage, which could help solar cells and photocatalytic devices achieve high efficiencies, the researchers say.
One form of upconversion uses two types of dye molecules, a sensitizer and an emitter. A sensitizer molecule absorbs low-energy photons to reach an excited state. An emitter then becomes excited through an energy transfer from the sensitizer. Through a series of energy transfers among emitter molecules, some get kicked up to an even higher excited state. When one of these super-excited emitter molecules then relaxes back to a lower energy state, it releases a single, high-energy photon.
Researchers want to use this process to allow solar cells or photocatalytic devices to harness a greater range of wavelengths in the solar spectrum. Unfortunately, oxygen interferes in upconversion. It quenches the energy transfer process, leading to short lifetimes for the dyes’ excited states. So researchers need to find ways to keep air away from these dyes, says Katharina Landfester of the Max Planck Institute for Polymer Research, in Germany.
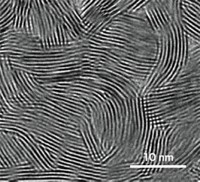
Landfester and her colleagues—including Stanislav Baluschev of Sofia University, in Bulgaria, and Anna J. Svagan of the University of Copenhagen—thought that mimicking the structure of a leaf might help solve this problem. Leaves encapsulate chlorophyll within chloroplasts to protect against oxygen damage during photosynthesis. In a similar way, the researchers thought that cellulose nanofibers derived from plants might encapsulate and protect light-sensitive dyes.
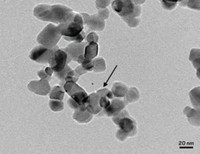
They and their colleagues packaged various dyes, five sensitizers and one emitter, into nanocellulose capsules about 1.2 μm in diameter and then embedded them into a matrix of cellulose nanofibers. The dyes were chosen to absorb light in the deep-red wavelength range and then emit green light after the upconversion process. The leaflike scaffold of the cellulose nanomaterials resulted in a flexible nanopaper that emitted blue-green light when excited with broadband light. The material continued this upconversion efficiently in air for at least an hour. As a comparison, the team embedded the same ensemble of dyes in a polystyrene film, which is more permeable to oxygen than cellulose nanofibers, and found that the upconversion lasted only seconds or minutes.
Felix N. Castellano, an inorganic photochemist at North Carolina State University who was not involved with the study, says the researchers have created a material that combats oxygen damage, “one of the biggest enemies to the photochemical upconversion process.” He calls the nanopaper a soft material that goes beyond traditional polymers with its ability to seal out reactive oxygen molecules, yielding a thin film valuable for integrating into devices.
The next step, Landfester says, is to increase the nanocellulose’s protective abilities by developing a method to actively destroy oxygen in the material.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter