Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Smell Receptors Sniff Out An Aldehyde In Its Hydrated Form
Olfaction: Some odorant receptors bind to aldehydes as geminal diols, a structure found in a nose’s moist environment
by Louisa Dalton
September 17, 2014

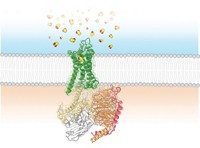
A rat cruising for dinner will undoubtedly sniff out aldehydes, common odorant molecules in foods and fragrances. But the chemical details of how rodent or other mammalian odorant receptors bind aldehydes are not well understood. Now scientists have discovered a new aspect of this process: Some odorant receptors recognize an aldehyde via its hydrated geminal diol form, which is different from the volatile carbonyl form (ACS Chem. Biol. 2014, DOI: 10.1021/cb400290u). The work suggests that some mammalian olfactory receptors depend on a watery carbonyl-to-diol conversion to sense aldehydes.
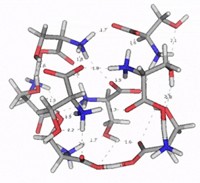
When an odorous aldehyde wafts through the air and lands in the nose’s watery mucus, it starts to waffle. In water, an aldehyde equilibrates between its carbonyl form and its hydrated, double hydroxyl version—a gem-diol. The two structures, says Kevin Ryan of the City College of New York, differ dramatically in shape and hydrogen-bonding capability. Yet both forms exist in moist mucus, so he wondered if any of the numerous aldehyde odorant receptors detected the hydrated form.
Ryan and his colleagues, including Stuart Firestein of Columbia University, suspected the answer would vary from one receptor to another. Odorant receptors that bind a diverse range of molecules probably wouldn’t discriminate on the basis of such a structural difference. But the researchers thought that receptors narrowly tuned to recognize only aldehydes might prefer the diol form.
To investigate the question, Ryan and Firestein decided to look at the gem-diol form of octanal, a citrusy aldehyde found in butter, oranges, and rice. However, octanal in water converts from the carbonyl to the diol form and back again, making it difficult to study the diol form on its own. So they made a fluorinated version, 2,2-difluorooctanal. This fluorinated aldehyde strongly favors the diol form when in water because of the electron withdrawing fluorines.
Then they tested the fluorinated compound on 24 odorant receptors that were activated only by aldehydes. About 40% of those receptors responded to 2,2-difluorooctanal, demonstrating that the gem-diol structure is one way that olfactory neurons respond to aldehydes, Ryan says.
Ryan’s group also collaborated with computational chemists at Hebrew University, in Jerusalem, to model a well-studied rodent odorant receptor called OR-I7. When they docked the gem-diol of octanal inside the receptor’s binding site, they found two tyrosines that could simultaneously hydrogen-bond with the gem-diol but not the carbonyl form. “It’s a structural hypothesis for future work,” Ryan says.
The experimental finding is especially important for computational modelers, says Hanns Hatt of Ruhr University Bochum, in Germany. With no hard structural data from X-ray crystallography yet available, many of the models of aldehyde odorant receptors created in the past few years have not considered the gem-diol as a ligand; he thinks future studies likely will.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter