Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Ribozyme Swaps Genes With A Flip Of A Switch
Gene Therapy: Researchers developed a ribozyme that targets and replaces genes only in the presence of a specific small molecule, offering a more controlled approach to gene therapy
by Erika Gebel Berg
October 10, 2014
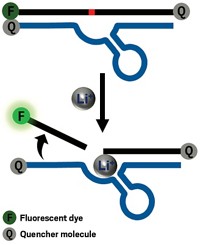
One serious limitation with current gene therapy strategies is a lack of control. When researchers deliver a gene to replace one linked to disease, there’s no way to turn on the therapeutic gene at the right place and the right time, limiting efficacy and potentially triggering serious side effects. In an effort to take control, researchers developed a catalytic RNA—a ribozyme— that exchanges genes only in the presence of a specific small molecule (ACS Chem. Biol. 2014, DOI: 10.1021/cb500567v).
Side effects arise in gene therapy when the therapeutic gene inserts itself into genes not linked to disease, possibly disrupting the function of essential genes or activating a cancer-causing gene. Seong-Wook Lee of Dankook University, in South Korea, and colleagues wanted to gain more control over gene splicing through a ribozyme they could turn off and on.
The researchers specifically looked at group I introns, which are large ribozymes that splice themselves out of strands of RNA. Scientists have converted group I introns into ribozymes that can bind to and replace a gene in a strand of target RNA. These so-called trans-splicing ribozymes carry a replacement gene at their tail ends and stitch it into the RNA strand in place of the target gene. In previous work, Lee developed a trans-splicing ribozyme that swapped a cancer-associated gene with a gene that damaged cancer cells (Mol. Ther. 2005, DOI: 10.1016/j.ymthe.2005.06.096).
To add an on-off button to such ribozymes, Lee and his group borrowed a feature of ribozymes found in some bacteria, fungi, and plants. Those catalytic RNAs regulate gene expression only when activated by a small molecule. Lee saw the opportunity to develop inducible gene therapy by building one of these so-called riboswitch features into his trans-splicing ribozyme.
The switch consisted of an aptamer, an RNA strand that can bind a certain small molecule. For this proof-of-principle experiment, the researchers selected an aptamer that binds theophylline, which has a structure similar to caffeine. The researchers analyzed their ribozyme’s crystal structure to identify areas that could accommodate an aptamer without compromising function. They wanted binding of the small molecule to shift the overall conformation of the ribozyme from an inactive to an active state. After testing multiple RNA sequences in test tubes, the researchers settled on one that reliably swapped genes in the presence of theophylline but not caffeine.
To test their approach in cells, the researchers designed their ribozyme to target the gene for human telomerase reverse transcriptase. Their ribozyme, when activated, would swap out this gene for one encoding a protein that would decrease the viability of the cells. They incorporated their ribozyme into a viral vector and then infected cultures of human cancer cells. Adding theophylline to the cultures reduced cell viability by more than 60%, but caffeine and buffer had little effect, demonstrating the selectivity of their strategy. Using reverse transcriptase polymerase chain reaction, the researchers checked the cultures for the replacement gene, finding four times as much of the product in theophylline-treated cultures as those receiving caffeine or buffer.
Yingfu Li of McMaster University, in Ontario, says using a small molecule to regulate gene splicing is a key and novel advance for the field. However, he says, an ideal strategy would link the small-molecule inducer to a disease. For example, the ligand could be a molecule specific to cancer cells. Lee is working toward this and has started to develop ribozymes with aptamers that work with a range of small molecules found in cells.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter