Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Polyribosomes May Act As 3-D Nanoprinters To Fabricate Vault Particles
Cell Biology: Researchers find evidence that the mysterious cellular particles assemble as they come off ribosomes
by Laura Cassiday
November 11, 2014

Three-dimensional printers fabricate a gamut of products such as medical devices, toys, and even specialty chocolates. Now a new study suggests that eukaryotic cells evolved a 3-D nanoprinter millions of years ago, in the form of polyribosomes. These clusters of ribosomes strung along a single messenger RNA appear to be responsible for the intricate 3-D assembly of a mysterious large, barrel-shaped protein complex called the vault particle (ACS Nano 2014, DOI: 10.1021/nn504778h).
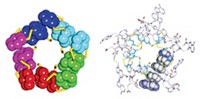
Although vault particles were discovered almost 30 years ago, their function and assembly remain mysterious. Found in most but not all eukaryotic cells, the 72-nm-long vault particle is composed of 78 copies of the major vault protein (MVP), tightly arrayed to form a capsulelike shell. The shell has two halves, each made of 39 MVPs aligned side by side. Packaged inside are two other proteins and some small RNAs. Because vaults protect their contents and are nonimmunogenic, researchers have explored the idea of filling the particles with drugs or other therapeutic molecules.
Jan Mrazek, a postdoctoral researcher in Leonard H. Rome’s lab at the University of California, Los Angeles, came up with the polyribosomes-as-3-D printers hypothesis while tinkering with these vault particles. In particular, he wanted to see if he could weaken the interactions among MVPs where the two halves come together so that the particles would be easier to open, enhancing their ability to package therapeutic molecules.
Mrazek made a series of mutations at the end of the MVP that forms the waist of the vault particle. He then expressed the MVP mutants in insect cells, which lack their own vaults, and observed the structures of the resulting complexes by electron microscopy. Mrazek found that four or five amino acid substitutions resulted in unstable vaults that separated into halves. But six substitutions produced a big surprise: large structures that appeared to be multiple vaults rolled up like a cinnamon roll.

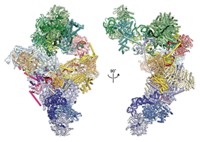
“I had never in my life seen anything like these rolls,” Mrazek says. “Normally, when you get misassembled proteins you see ugly tangles, but these were so symmetric.” Mrazek concluded that the rolls must reflect structural intermediates during the vault assembly process. Ribosomes moving along an mRNA normally synthesize individual protein strands, which then come off the ribosome and fold into their 3-D shapes. On the basis of the proposed helical geometry of the polyribosome, Mrazek hypothesized that as an individual MVP molecule gets translated by a ribosome in the cluster, it could form a dimer with the MVP synthesized by the ribosome next to it. As they’re formed, adjacent dimers could then arrange side by side to form the vault particle. The vaults take shape bit by bit as the dimers are completed and come off the polyribosome, much like a 3-D printer might build up the layers of a plastic object. The sixth mutation in the MVP somehow disrupts the pinching off of the vault particle after the 39th dimer assembles, resulting in the long, rolled-up vaults.
In further support of the 3-D nanoprinter hypothesis, Mrazek found that vaults formed even when very low concentrations of MVP were translated in solution in a tube. In such a dilute solution, it would be difficult for all the MVP particles to self-assemble into vaults, so they must be coming together as the protein strands are synthesized. Also, using electron microscopy, the researchers observed individual vaults or rolls attached to polyribosomes from cells expressing wild-type or mutant MVP, respectively.
Although this is the first example of a protein complex that the polyribosome assembles during translation, Rome doesn’t think vault particles are unique in their mode of fabrication. “We think that the cell doesn’t do such elegant biochemistry for one structure,” he says. “There must be many other structures in the cell that take advantage of the geometry of the polyribosome.”
Julio Ortiz at the Max Planck Institute of Biochemistry, in Germany, says that the model opens new avenues for the 3-D structural analysis of vault assembly. “This paper shows an interesting example of the need for spatially coordinated synthesis of nascent chains for the correct assembly of a large macromolecular complex,” he says. However, Ortiz notes that there are other proposed geometries for polyribosomes, and not all of them are consistent with the nanoprinter model presented in the paper.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter