Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
3-D Printing
Printing 3-D Conductive Materials With The Help Of Ionic Liquids
Materials: The technique could allow engineers to print conductive components for batteries or even tissue scaffolds
by Neil Savage
November 19, 2014
\
\
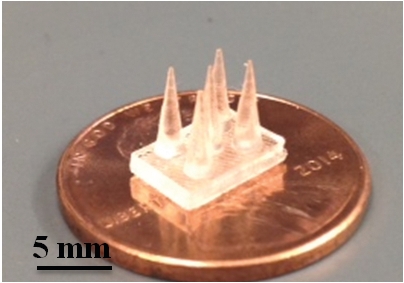
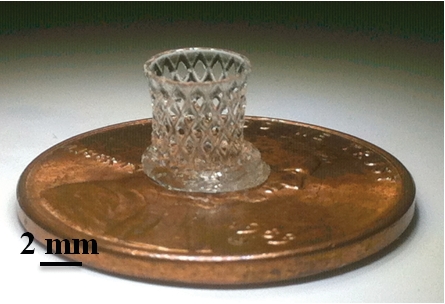
BUILDING BLOCKS
Using stereolithography and a polymer ionic liquid, researchers printed objects of various shapes, including cones (top) and a cylindrical lattice known as a hyperboloid (bottom), each 8 mm in height.
Three-dimensional printing could revolutionize how industries ranging from aerospace to fashion build products. So scientists have tried to expand the materials that can be used in such manufacturing beyond simple plastics and metals. Now a group has printed 3-D objects from a conductive polymer made using an ionic liquid (ACS Macro Lett. 2014, DOI: 10.1021/mz5006316). With this material, 3-D printers could build ion-conducting membranes for batteries and fuel cells, electromechanical actuators, or scaffolds for growing cells.
Chemist Timothy E. Long, mechanical engineer Christopher B. Williams, and their team at Virginia Tech built millimeter-sized objects with the ionic liquid 4-vinylbenzyl trioctyl phosphonium bis(trifluoromethanesulfonate)imide. They mixed it with a diacrylic monomer, such as 1,4-butanediol diacrylate or poly(ethylene glycol) dimethacrylate, both of which can be cured by ultraviolet light. When hit with light, the diacrylic monomers and phosphonium ions formed cross-linked polymer networks.
The researchers used stereolithography to create their 3-D printed objects, exposing a layer of the ionic liquid mixture to UV light in a pattern determined with a computer-aided design system. They then coated the cured polymer layer with more mixture and shone light on it, repeating the process layer by layer until they built up their desired object.
The objects they printed had features as small as 25 µm. These small features would be compatible with growing human cells; the conductivity of such a polymer scaffold could help grow tissues for skin or bone grafts.
Long believes this is the first example of printing with an ionic liquid. Though the team didn’t create any functional devices, they did test the conductivity of their objects and found that the cured polymer retained the conductive properties of the ionic liquid.
Other researchers have worked with polymer ionic liquids outside of 3-D printing, but those materials consisted of nitrogen-based ionic liquids, including ammonium or imidazolium ions. But phosphonium ionic liquids, which have only recently become widely available, have higher thermal stability, remaining intact to at least 300 °C, compared with 200 °C for ammonium ionic liquids. Higher stability is important in some high-temperature applications, such as in aerospace. Even if the devices don’t need to survive those temperatures, their enhanced stability means they’ll last longer at lower operating temperatures.
Long’s team plans to explore variations of the phosphonium ionic liquid material, trying different cross-linking monomers or different charge concentrations. These variations could change characteristics of the printed materials such as mechanical and conductive properties.
Joseph M. DeSimone, a chemical engineer at the University of North Carolina, Chapel Hill, calls the work both very important and very cool. “I think this particular class of materials will trigger many ideas in a whole host of applications including electrochemical cells, biological systems, and separation media,” he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter