Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
A New Link For Aromatic Polymers
Polymer Science: Chemists have designed a direct method for preparing poly(o-arylene)s for the first time
by Stephen K. Ritter
December 29, 2014
Chemists in Japan have developed the first direct process for synthesizing poly(o-arylene)s, an achievement that provides new structural diversity to the polyphenylene class of polymers (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja5112207).
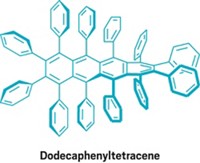
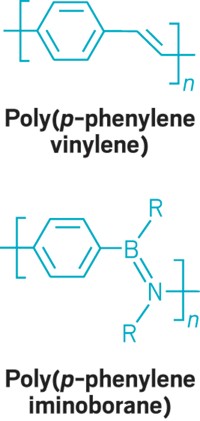
Direct synthesis of poly(o-arylene)s has been missing from the aromatic chemistry tool kit for a long time. The discovery of arynes, which are six-membered rings containing a carbon-carbon triple bond, dates to 1902. Polyarylenes in which aromatic rings are successively connected through the para and meta positions of the rings are known, but the ortho-linked versions had remained elusive.
The ortho linkages give the polymers a different helical shape and will likely provide different stimulus-response behaviors than phenylenes linked through other ring positions, the researchers believe. These properties are expected to open up new possibilities for developing nanocarbon materials for chiral catalysis, thin films that selectively reflect circularly polarized light, and building blocks for supramolecular-structured materials to make electronic devices and chemical sensors.
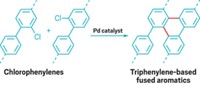
Chemists indirectly made poly(o-arylene)s previously by polymerizing bicyclic alkenes and following up with a dehydration step (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja502073k). In the new work, Yoshihide Mizukoshi, Koichiro Mikami, and Masanobu Uchiyama of the University of Tokyo first treated an aryl trimethylsilyl triflate precursor with fluoride ion to generate an intermediate aryne. The polymerization was then mediated by a copper(I) reagent that directs ortho linkages, yielding poly(o-arylene)s up to 100 units long via a chain-growth polymerization mechanism.
“Considering the more than 100-year history of aryne chemistry and its formal resemblance to alkynes, it is rather strange that no one has ever succeeded in realizing addition polymerization of this highly reactive species,” says Eiji Ihara, a polymer chemist at Ehime University, in Japan, who has explored myriad potential ways to polymerize arynes (Macromolecules 2005, DOI: 10.1021/ma048668b).
Ihara thinks the reason for the previous lack of success stems from arynes being too reactive for controlled polymerization, in contrast to olefins and alkynes, whose controlled polymerizations are well-established. “In that context, the achievement of Uchiyama’s group is quite significant, adding a totally new member to a family of monomers,” Ihara says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter