Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Computational Models Of Multistep Reaction Are Called So Flawed They Are ‘Not Even Wrong’
Reaction Chemistry: Study reveals mechanism theoretical work didn’t predict
by Stu Borman
March 5, 2015
| A version of this story appeared in
Volume 93, Issue 10

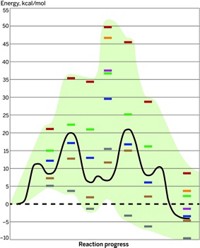
Chemists often use computational methods to predict reaction pathways and energies, but some researchers question their usefulness because the models sometimes produce highly variable, head-scratching results. Now, a detailed case study of a multistep organic reaction attacks the utility of computer modeling of that reaction in an unusually blunt way.
The authors conclude that the reaction mechanism is a simple one that undergraduates could guess and that a complex mechanism predicted by years of computational studies is “not even wrong”—so flawed and off-base that calling it incorrect is too kind.
The study by R. Erik Plata and Daniel A. Singleton of Texas A&M University focuses on the Morita-Baylis-Hillman (MBH) reaction, in which an electron-deficient alkene, a nucleophile catalyst, and an aldehyde react to form an allylic alcohol (J. Am. Chem. Soc. 2015, DOI: 10.1021/ja5111392). The researchers conducted a wide range of experiments to nail down the reaction’s mechanism and energetics conclusively for the first time.
Computational studies on the reaction have been “arguably more misleading than enlightening,” they conclude. “It is not clear to us that any reliably accurate information that was not already apparent from experiment could have been garnered from calculations.” The most notable theoretical prediction was that the reaction involves a complex “proton-shuttle” pathway, but experiments find the mechanism to be a simple acid-base process.
Chemical theoretician Kendall N. Houk of UCLA says the paper “is full of profound insights, including one that astute computational chemists are all familiar with”—that they cannot currently use standard computational methods to predict some features of solution reactions. Theory, he says, “is still not capable, and may never be capable, of predicting what happens when many chemicals, four in this case, are mixed in solution.”
The study’s harsh verdict on theory is based only on an analysis of the MBH reaction. Nevertheless, theoretical “studies of complex multimolecular polar reactions in solution should be undertaken and interpreted only with extreme care,” the paper suggests.
Experimental chemist Tyler McQuade of Florida State University says the study “provides a call to arms for our community.” Experimentalists and theoreticians, he says, “need to continue developing methods and testing their veracity with a skeptical eye.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter