Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Scientists Search For Small Molecules To Treat Autism Spectrum Disorder
ACS Meeting News: Thanks to new genetic discoveries and animal models, researchers are finally making headway toward therapies
by Bethany Halford
April 27, 2015
| A version of this story appeared in
Volume 93, Issue 17

Autism spectrum disorder, a group of complex brain development disorders, affects approximately 3.5 million people in the U.S. alone. The U.S. Centers for Disease Control & Prevention estimates that one in every 68 children will be diagnosed with autism and that in the past 10 years diagnoses have more than doubled.
There was a time not so long ago when scientists had no idea what caused autism. But in recent years researchers have identified genetic mutations that can be linked to some disorders on the autism spectrum. They also think there are some genetic mutations that carry a risk of autism when combined with environmental factors.
The upshot of these findings is that scientists are beginning to tease out potential pathways for treating the disorders or some of their more debilitating symptoms, such as anxiety and impaired social interaction. At last month’s American Chemical Society national meeting in Denver, a handful of these researchers gathered at a symposium in the Division of Medicinal Chemistry to discuss the challenges in developing small molecules to treat autism spectrum and related disorders and the progress they’re making toward that goal.
Most of what we currently know that helps kids with autism are behavioral treatments, said Jeremy Veenstra-VanderWeele, a psychiatrist at Columbia University. As far as medicinal treatments go, there are some drugs, such as risperidone and aripiprazole, which seem to help with irritability, agitation, and aggression symptoms in people with autism spectrum disorder. But beyond that, he said, “what we know at this point is, unfortunately, quite little.”
Fortunately genetics, Veenstra-VanderWeele said, is beginning to provide some clues for autism researchers. In just the past five years, he said, scientists have gone from not knowing about any autism-related genes to finding more than 20 that have been linked to the disorder. Those findings have led researchers to start studying the neurobiology underlying autism. “It really gives us an opportunity to work from a point of knowledge as opposed to conjecture,” he said.
Genetics, for example, has been particularly illuminating in the study of a condition called Rett syndrome. Rett syndrome is a disorder that almost exclusively affects girls, who seem to develop normally until about 18 months of age, when they begin to lose motor control and socialization skills. Girls with Rett syndrome develop some autistic behaviors.
Rett syndrome occurs as a result of a mutation in the MECP2 gene, which contains the instructions for making methyl cytosine binding protein 2, or MeCP2. This transcription factor controls the activity of many other genes, so when the MECP2 gene doesn’t work properly it can foul up other genes required for brain development.
“Currently there are no treatments for this disorder,” said David M. Katz, a professor of neuroscience at Case Western Reserve University School of Medicine.
Katz’s research team has been studying mice that have the same MECP2 mutations that produce Rett syndrome in humans. The mouse model, he says, is a good one because the mice exhibit many measurable behaviors that girls with Rett syndrome have. For example, they have irregular breathing patterns, sometimes hyperventilating and sometimes not breathing at all for unusually long periods of time. Scientists can quantify these abnormalities and see if therapies change them.
“Rett syndrome is really a problem of how neurons communicate with one another,” Katz explained. “It’s not a problem of degeneration of nerve cells or death of nerve cells, like you would have with Alzheimer’s disease. This is actually quite hopeful because it means the structure of the brain is intact.” It also suggests that it’s theoretically possible to reverse Rett syndrome in adult patients.
“Until the day comes when we can actually fix the gene, we believe that it’s going to take a cocktail approach—more than one drug—because multiple different pathways are disregulated when the MECP2 gene is mutated,” Katz explained. “We don’t think that any one drug is going to address all of the symptoms of the disease.”
One pathway Katz and colleagues have been studying with the aim of improving communication between neurons involves the N-methyl-
Katz said that the trial will tell researchers a lot about whether the NMDA receptor is a viable target for treating Rett syndrome. “We’re cautiously very optimistic that we will be able to bring treatments to the clinic at least for some of the symptoms of the disorder within the next five years or so,” Katz said.
AstraZeneca also has been targeting the NMDA receptor for treating Rett syndrome. Robert J. (Joe) Mather, a neuroscientist at the company, spoke about two compounds, remacemide and lanicemine, which have proven particularly effective at reversing breathing abnormalities and improving motor skills in mice with Rett syndrome.
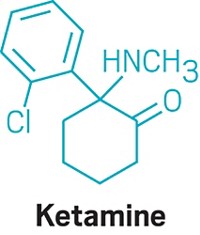
Remacemide is a compound that was developed to treat epilepsy, Mather said, but the compound was put aside because of a change in strategy. “This drug has basically been sitting on the shelf,” he said. Similarly, lanicemine was developed to treat depression but had mixed results in clinical trials. Like ketamine, both molecules target NMDA receptors, but they seem to have a higher tolerability than ketamine. “If you gave a comparable dose of ketamine,” Mather said, “patients would basically be high.” Remacemide is currently in preclinical testing with the goal of bringing it to patients with Rett syndrome.
Beyond Rett syndrome, scientists are studying pathways associated with symptoms of disorders officially within the autism spectrum. For example, Kyle A. Emmitte, associate director of medicinal chemistry at the Vanderbilt Center for Neuroscience Drug Discovery, has been developing selective inhibitors of metabotropic glutamate receptors to treat anxiety and obsessive compulsive behaviors associated with autism.

Researchers have been looking to find which receptors from the family of eight are linked to these symptoms. So far, they know that two—mGlu2 and mGlu3—have definite links. Emmitte’s group has been working to tease out differences between these two by developing compounds that selectively block mGlu3 and not mGlu2.
Emmitte spoke about the medicinal chemistry process of synthesizing and studying approximately 250 compounds, eventually arriving at VU0650786, which is selective for mGlu3. In rodent models of obsessive compulsive disorder, the compound works similarly to compounds that target both mGlu2 and mGlu3. “We haven’t proven that mGlu2 is not involved in these disorders,” Emmitte said, “but we’ve proven that an mGlu3-selective compound works in these models.”
Perhaps one of the biggest hurdles in autism research is getting drugmakers on board to develop new therapeutics. “Because the field is relatively complex, most major pharmaceutical makers are not developing small molecules for autism spectrum disorder,” said Carrie K. Jones, director of in vivo and translational pharmacology at Vanderbilt Center for Neuroscience Drug Discovery.
One major reason for this reticence, Jones thinks, has been the lack of good animal models for autism. If scientists were able to show there was a standardized animal model for autism that gave reproducible results, it would certainly give drugmakers more confidence that they’d see similar results in people.
To that end, Jones and several other researchers have been working with the autism advocacy group Autism Speaks on what’s known as PACT, or Preclinical Autism Consortium for Therapeutics. Jones and her PACT colleagues have been doing extensive work to validate rat and mouse models of autism. After two years of research, they have determined that rodents with one of four different genetic mutations are good models of a range of facets of autism spectrum disorders.
Since presenting her work at the ACS meeting, Jones told C&EN she’s been contacted by five different pharmaceutical companies.
Emmitte, who also organized the symposium, said those kinds of interactions are exactly what he was hoping to achieve with the meeting. “We’re still in the early days of understanding this disease,” he told C&EN. “But I wanted people to know that there’s some great science going on in an area where the unmet medical need is really high.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter