Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Styrene Produced In One Step
Industrial Organic Chemistry: New catalyst enables single-step synthesis of styrene from benzene and ethylene
by Stu Borman
April 27, 2015
| A version of this story appeared in
Volume 93, Issue 17
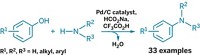
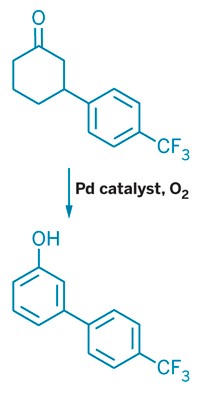
A new catalyst makes possible a long-standing research goal—synthesizing styrene from benzene and ethylene in a single step. Styrene, used for fine chemicals synthesis and plastics and elastomer preparation, is currently produced globally in the 20 million-ton-per-year range, so a more efficient method could result in significant cost savings. It’s generally made by using AlCl3 (with HF) or zeolites to convert benzene and ethylene to ethylbenzene, followed by dehydrogenation to styrene. Now, T. Brent Gunnoe of the University of Virginia, Thomas R. Cundari of the University of North Texas, and coworkers have identified a rhodium catalyst that does the trick in one step (Science 2015, DOI: 10.1126/science.aaa2260). Their approach converts benzene, ethylene, and a Cu(II) reagent to styrene plus a Cu(I) compound, with 100% styrene selectivity. Although a rhodium catalyst might be too expensive for practical industrial use, Gunnoe believes it’s a major step toward a more efficient industrial process and notes that its selectivity is unprecedented. “If they can ultimately recycle the copper oxidant with oxygen, the commercial potential looks high,” comments Karen Goldberg of the University of Washington.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter